In a groundbreaking development that challenges longstanding paradigms of cellular aging, a recent study unveils a remarkable mechanism by which embryonic stem cells (ESCs) maintain their youthful vigor and immortality across infinite cell divisions. Published in Cell Research, the study conducted by Wang, Fu, Sun, and colleagues dissects the enigmatic process that allows ESC lineages to evade the usual fate of senescence—delivering fresh insights into the biology of aging, regeneration, and potential future therapies.
For decades, the question of whether all cells inevitably age has posed a biological conundrum. While most somatic cells accumulate damage over their lifespan, leading to aging and eventual death, ESCs have stood apart as oddities capable of passing through countless in vitro divisions without apparent decline in functionality. This capacity for indefinite self-renewal underscores their critical role in development and offers immense promise for regenerative medicine, yet a detailed understanding of the underlying cellular mechanisms had remained elusive—until now.
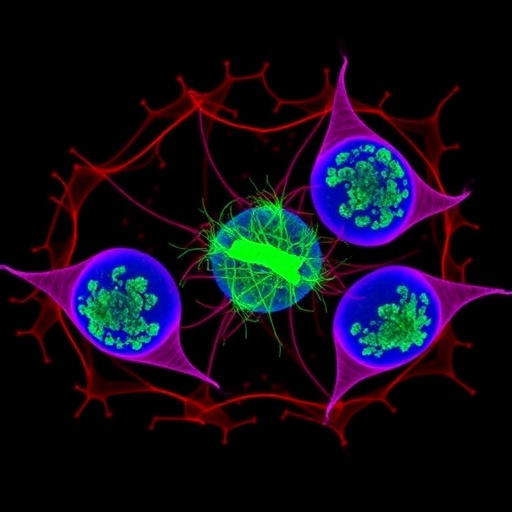
Leveraging advanced long-term live-cell imaging techniques that allow continuous monitoring of individual ESCs, the researchers traced the dynamic fate of these cells over extensive culture periods. Their observations revealed something profoundly unexpected: the stem cell lineage periodically enters a distinctive “two-cell-like” state characterized by expression of molecular markers typically exclusive to two-cell stage embryos. This transient program is not merely a marker of identity but functionally pivotal for lineage maintenance.
During this two-cell-like state, ESCs undergo asymmetric cell division, a fundamental process in biology where one parent cell produces two daughter cells with distinct fates. Far from a simple young-to-old split, these divisions serve a strikingly targeted role—specifically partitioning accumulated DNA damage asymmetrically. One daughter inherits a higher burden of damaged DNA and is effectively earmarked for elimination, presumably preventing damage accumulation throughout the stem cell pool. Meanwhile, the other daughter cell escapes with fresher genomic integrity and reverts seamlessly to the canonical pluripotent ESC state.
This asymmetric partitioning acts as a quality control mechanism within the ESC population. As DNA damage is unevenly segregated and disposed of through elimination of the compromised lineage, the remaining stem cells effectively ‘reset’ their biological clock, manifesting signs of cellular rejuvenation. This process reveals a sophisticated way that ESCs manage the inevitability of molecular wear and tear, maintaining lineage health across generational boundaries.
Crucially, the progeny of these decluttered, low-damage ESCs exhibit not only reduced markers of DNA damage but also enhanced chimeric competence—a measure of their functional potential in contributing to embryonic development. The elevated chimeric efficiency reflects real-world implications of this rejuvenation, hinting that the ESCs emerging from asymmetric divisions retain superior regenerative capabilities.
This discovery fundamentally reframes our understanding of stem cell biology. Rather than being immortal by default, ESC immortality appears to be an actively maintained state, reliant on sporadic entry into a two-cell-like program coupled with meticulously orchestrated asymmetric divisions. Instead of indiscriminately diluting damage through mere replication, these cells employ targeted, strategic damage segregation to sustain their youthfulness.
From a mechanistic standpoint, the study sheds light on the molecular underpinnings initiating and regulating this two-cell-like state transition within ESC cultures. Emerging evidence suggests differential gene expression networks akin to zygotic genome activation may be recapitulated temporarily in vitro, aligning ESC behaviors with developmental milestones previously thought unique to early embryos. The transient expression of two-cell stage markers like Zscan4 defines this unique cell state, acting as hallmarks guiding the asymmetric fate decisions.
Moreover, this research carries significant implications for aging biology at large. By elucidating intrinsic cellular strategies for damage management and self-renewal, it opens new avenues for understanding how somatic cells might be coaxed towards rejuvenation. Could the molecular cues that trigger this two-cell-like state be harnessed therapeutically? Might asymmetric division be exploited to rid aged cell populations of damage accumulation? The findings invite provocative questions addressing the boundaries between aging, regeneration, and cancer biology.
Methodologically, the work exemplifies the power of integrating cutting-edge live imaging with molecular profiling to tease apart complex cell fate dynamics. Traditional static snapshots fail to capture the transient, probabilistic nature of this rejuvenation mechanism. By following single cells through multiple divisions over long periods, the researchers painted a dynamic portrait of an enigmatic cellular lifecycle rarely glimpsed in vitro or in vivo.
Beyond the laboratory, these insights nod toward translational possibilities. ESCs are central to a variety of regenerative therapies and disease modeling approaches. Understanding how to maintain or even enhance their ‘youthful’ quality could improve the efficacy and safety of cell-based treatments. Preventing the accumulation of genomic damage in therapeutic stem cell populations is vital for minimizing risks such as tumorigenic transformations.
In summary, Wang et al.’s discovery that ESCs repeatedly access a two-cell-like state to asymmetrically segregate DNA damage and subsequently rejuvenate reshapes fundamental concepts of cellular immortality. Their elegant model integrates developmental biology, aging science, and stem cell technology into a cohesive framework illuminating how cells can escape the upward spiral of damage accumulation.
Future research will undoubtedly delve deeper into the signaling pathways and epigenetic landscapes governing this process, exploring whether similar mechanisms operate in other stem cell types or differentiated cells. Understanding the triggers and regulators of the two-cell-like state could also uncover new biomarkers for stem cell quality and vitality.
Intriguingly, the role of asymmetric division in ESCs may parallel phenomena in tissue stem cells of adult organisms, which balance self-renewal and differentiation while managing cellular damage. This study provides a rare window into a natural rejuvenation system, one that may eventually inspire novel anti-aging and regenerative strategies.
Ultimately, this research challenges us to reconsider how life sustains itself at the cellular level amidst an environment of constant molecular degradation. The elegant solution of selective damage segregation embodied by ESCs is reminiscent of a timeless cellular balancing act—preserving youth by relegating the old and impaired to oblivion, while clearing the way for rejuvenated successors.
The elucidation of asymmetric division in a two-cell-like state as a rejuvenation strategy is a testament to nature’s ingenuity. It highlights a hidden regenerative rhythm within stem cell biology that might one day unlock new therapies for aging and disease, revolutionizing our grasp of life’s endurance.
Subject of Research: Embryonic stem cells, cellular aging, asymmetric cell division, cellular rejuvenation
Article Title: Asymmetric division in a two-cell-like state rejuvenates embryonic stem cells.
Article References:
Wang, X., Fu, H., Sun, Q. et al. Asymmetric division in a two-cell-like state rejuvenates embryonic stem cells. Cell Res (2026). https://doi.org/10.1038/s41422-026-01221-z
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41422-026-01221-z
Tags: asymmetric cell divisionbiological conundrum of agingcellular immortality mechanismscellular senescence avoidanceembryonic stem cell agingESC lineages and functionalityfuture therapies for aginginsights into aging biologylong-term live-cell imagingmolecular markers in stem cellsregenerative medicine potentialstem cell self-renewal processes