Credit: Juan Alegre-Requena/Colorado State University
If pharmaceutical chemists are the drug hunters who discover new medicines, scientists like Andrew McNally and Robert Paton are the armorers – the deft creators who arm drug hunters with the sharpest tools.
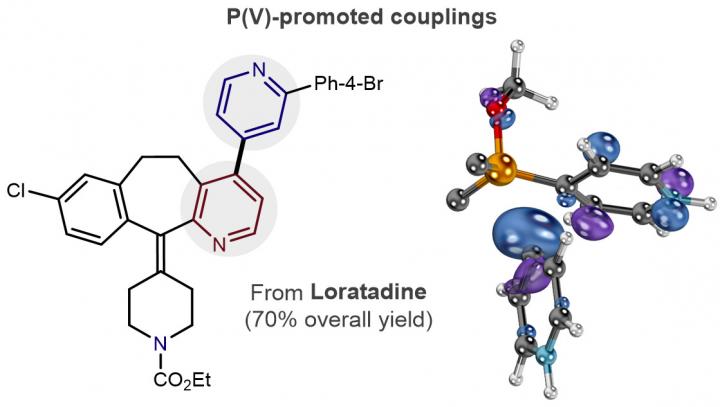
The pair of Colorado State University organic chemists have forged a powerful new such tool for drug hunters – a simple, elegantly designed chemical reaction that could fling open an underexplored wing of biologically relevant chemistry. Their contribution, detailed in the journal Science Nov. 16, could be a shot in the arm for the discovery of new drugs.
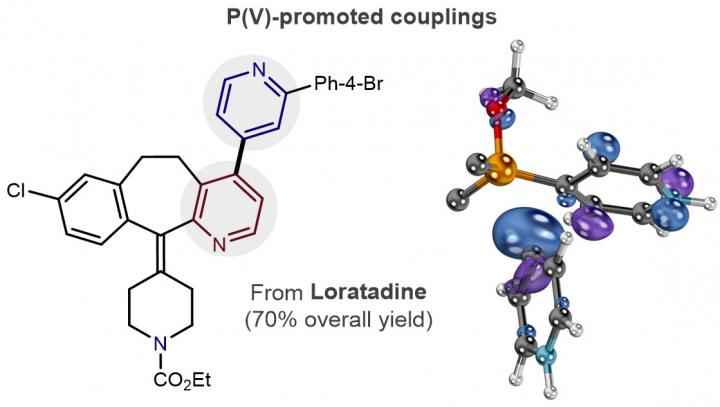
Assistant Professor McNally, a synthetic chemist, and Associate Professor Paton, an expert in computational chemical design, joined forces to create a new carbon-carbon bond reaction that's fundamental to how small-molecule drugs are made and discovered. The reaction uses phosphorus, rather than a commonly used transition metal, to stitch together molecular rings called pyridines. The lack of an accessible chemical reaction for coupling pyridine rings had been a deficiency in the field of drug discovery.
The new reaction, created in McNally's lab, is analogous to the well-known palladium-catalyzed cross-coupling reaction, which makes carbon-carbon bonds using the transition metal palladium as a point of contact. Palladium-catalyzed reactions, which were the subject of the 2010 Nobel Prize in Chemistry, have been used for 30-plus years in pharmaceutical labs as the workhorse chemistry for coupling benzene rings. Benzene coupling is a foundational reaction in many pharmaceutically active compounds, from which thousands of drugs today – painkillers, antimalarials, contraceptives – were first synthesized in laboratories.
But the palladium-catalyzed reaction, for which the late CSU chemist John Stille was a major innovator in the 1970 and 80s, does not work as well for coupling pyridine rings. Coupled pyridine rings are a potentially valuable pharmacophore, or chemical part known to interact with a biological system – the basis for how drugs interact with the body. McNally's creation thus allows for easy construction of traditionally difficult-to-make chemical compounds that are known biological targets. They offer potential for discovery of drugs for diseases old and new – a new arsenal of tools previously out of reach.
"A major goal for our lab has always been for anyone in a pharmaceutical setting to go into the lab and try out our chemistry," McNally said. "If people can pick this up and start to use it to discover drug leads, that would be an incredible win. We have used the transition metal chemistry for many years, but to get a new approach in there has been quite hard. We've tried to make this as easy as possible."
Collaborating with Paton's lab was integral to the discovery of the new reaction, McNally said, because experimentation alone could not have yielded their resulting model. Paton specializes in quantum chemistry, using it to rationally design new chemical structures to carry out specific tasks. Through these methods, Paton and his team validated the use of phosphorus, and followed the mechanism by which the challenging pyridine coupling is orchestrated.
"This is the first study we know of that gives us a complete understanding of how these bonds are made," McNally said. "People had viewed these phosphorus-mediated reactions as somewhat esoteric, with no practical significance. The model we developed has also allowed us to develop other reactions that will be valuable to the pharmaceutical industry that are ongoing in our laboratory."
Paton says he hopes medicinal chemists use this new chemistry to develop libraries of compounds with phosphorus-catalyzed pyridine couplings, and that these libraries could open doors for new drug treatments.
"We want to give people reliable methods they can use every day to make important molecules," McNally said.
###
Media Contact
Anne Manning
[email protected]
970-491-7099
@ColoStateNews