Credit: National Institute of Allergy and Infectious Diseases
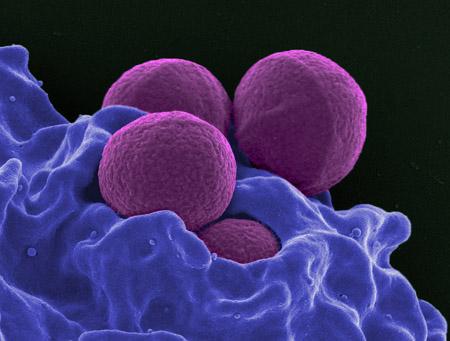
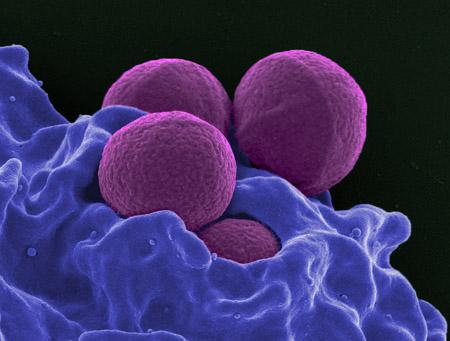
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterial scourge. As its name suggests, MRSA is resistant to most common antibiotics and thus difficult to treat, particularly in children where it commonly causes complicated skin and skin structure infections.
In a randomized, controlled clinical trial — the first of its kind — a multi-institution research team reports that daptomycin, part of a new class of antibiotics currently approved only for use in adults, is effective and well-tolerated in children. The findings are published in the March 2017 issue of Pediatrics.
"The safety and efficacy of intravenous daptomycin was comparable to standard-of-care IV antibiotics used for hospitalized children, usually vancomycin or clindamycin for MRSA and cefazolin for methicillin-susceptible strains of S. aureus," said first author John Bradley, MD, professor of clinical pediatrics, co-chief of the Division of Infectious Diseases at UC San Diego School of Medicine and director of the Division of Infectious Diseases at Rady Children's Hospital-San Diego.
"Daptomycin should provide a safe and effective alternative to vancomycin, clindamycin or linezolid for IV treatment of invasive MRSA skin infections. Concerns for vancomycin renal toxicity and clindamycin antibiotic resistance were not present. There was no evidence of daptomycin toxicity in the trial."
The Food and Drug Administration is currently reviewing whether to approve daptomycin use in children.
MRSA infections are commonly associated with patients in hospitals and nursing homes whose immune systems are weakened, but community-associated MRSA (CA-MRSA) is widespread, readily transmitted at daycare centers, playgrounds and in schools where children have frequent skin-to-skin contact, share toys that have not been cleaned and are more likely to have scrapes, abrasions and bites that offer potential infection entry points.
CA-MRSA usually causes skin infections but can lead to more serious consequences, such as pneumonia and infections of bones and joints. Daptomycin is active against MRSA and was approved for use in adults in 2003 for treatment of skin and skin structure infections, and for bloodstream infections three years later.
The new study was a prospective, randomized, investigator-blinded study that included more than 250 daptomycin-exposed children, ages 1 to 17, to document safety and efficacy of the antibiotic in treating pediatric skin and skin structure infections. Dosing was based on adult experience, but researchers found that the younger the child, the more quickly their bodies eliminated daptomycin. Thus pediatric doses increased as the age of the research participants decreased.
"Most news these days is about the declining utility of antibiotics as microbial resistance becomes more widespread and intractable," said Bradley. "These findings are encouraging. Daptomycin appears to be a suitable, once-a-day alternative to existing antibiotics with harsher side effects."
###
Co-authors include: Chad Glasser, Hernando Patino, Minjung Yoon, Diane Anastasiou, Dominik Wolf, and Paula Bokesch, Merck; Sandra L.R. Arnold, University of Tennessee; Antonio Arrieta, Children's Hospital of Orange County; Blaise Congeni, Akron Children's Hospital, Ohio; Robert Daum, University of Chicago MRSA Research Center; and Tsoline Kojaoghlanian, Children's Hospital at Montefiore, Bronx, NY.
Disclosure: Bradley, Arnold, Arrieta, Congeni, Daum and Kojaoghlanian were study site principal investigators whose respective employers received institutional research funding from the study sponsor, Merck. Additionally, UC San Diego received funding from Cubist Pharmaceuticals, now part of Merck, to advise Cubist on clinical trial design.
Media Contact
Scott LaFee
[email protected]
858-249-0456
@UCSanDiego
http://www.ucsd.edu