Expanding DNA by adding a synthetic base pair holds the promise of producing unique proteins and peptides for new medicines and technologies. But now, research led by the Department of Chemistry at Case Western Reserve University shows the expansion of the genetic code carries an unintended consequence.
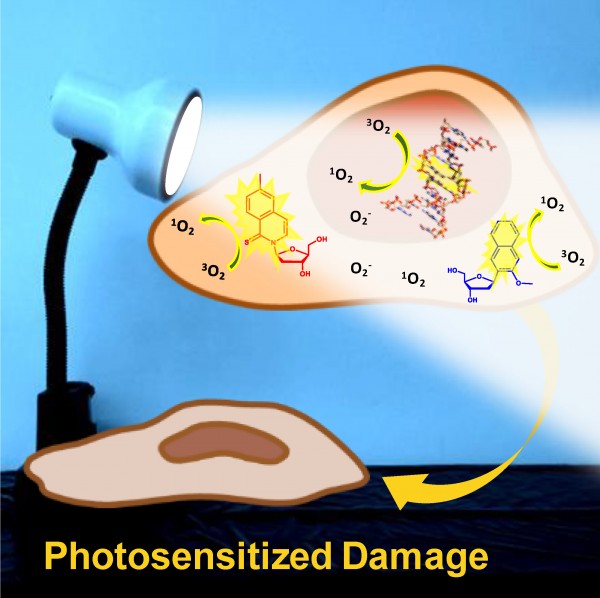
Scientists found that the synthetic bases d5SICS and dNaM, used to increase the number of DNA bases from the four that occur naturally in all organisms to six, also makes cells so sensitive to near-visible ultraviolet light that the light becomes toxic to cells.
“The light-induced chemistry of these synthetic bases has a high potential of damaging DNA and other biomolecules in the cell,” said Carlos Crespo-Hernández, associate professor of chemistry and principal investigator of the study.
The researchers warn that sunlight and even lighting in a lab can induce phototoxic side effects, which limits cell viability and may lead to genetic mutations. But the team also found a potential way to take advantage of the otherwise deleterious effects: In skin cancer cells carrying the d5SICS synthetic base, exposure to near-visible ultraviolet light significantly decreased cancer proliferation.
The research was recently published in the Journal of the American Chemical Society, and can be found using the doi: 10.1021/jacs.6b06822.
Crespo-Hernández began this research after learning that Floyd Romesberg, a professor at Scripps Research Institute, made the first known semisynthetic organism to incorporate and replicate a six letter genetic alphabet.
Romesberg expanded the DNA in a modified strain of the bacteria Escherichia coli by adding the d5SICS·dNaM base pair. Because the organism now has six instead of four bases, it can potentially produce proteins and peptides that can’t be made naturally. Romesberg’s work was named one of the top scientific breakthroughs of 2014 by Science magazine, winning the ‘people’s choice’ for #1 scientific breakthrough of the year.
Crespo-Hernández quickly recognized that the synthetic bases may engender phototoxicity in cells. One of his lab’s research trusts is on the photochemistry of DNA’s natural bases as well as of modified bases exhibiting light-induced toxicity with the potential to treat cancers.
He tapped PhD student Marvin Pollum to lead the investigation. Pollum and undergraduate researcher Brennan Ashwood from his group initially used a state-of-the-art laser system to “watch” how d5SICS and dNaM deal with the energy absorbed from near-visible ultraviolet light.
“The synthetic bases can’t get rid of the extra energy,” Pollum said. Most of the energy is essentially trapped, and can instead induce chemical reactions and/or generate reactive oxygen species (ROS), which are known to damage biomolecules and DNA, he explained.
Pollum sent samples to Steffen Jockusch, associate research scientist in the Department of Chemistry at Columbia University, who found that the synthetic bases produce high amounts of an ROS called singlet oxygen—as much as 100 times more than produced by the most reactive natural base under ultraviolet light.
Ultraviolet light can damage the natural bases in DNA, but cells have mechanisms to repair most of the damage. To test what happens in cells containing the synthetic d5SICS base, Pollum teamed with Minh Lam, assistant professor in the Department of Medicine at Case Western Reserve.
They incorporated incrementally increasing amounts of the base d5SICS into a skin cancer cell line.
Pollum exposed half the cells to near-visible ultraviolet light, with wavelengths ranging from 350 to 410 nanometers. After three days, cells lacking the synthetic base and cells with the synthetic base but not exposed to the light showed no difference in ROS presence or proliferation.
But cells carrying the synthetic base and exposed to the light showed more ROS and significantly less proliferation. The higher the concentration of d5SICS among these cells, the less the cells proliferated following a brief period of light exposure.
Further research is needed to determine whether the synthetic bases that are transported into the cancer cells are further incorporated in the cells’ DNA or are floating in the cytoplasm, and whether one makes the cell more susceptible to light than the other, Crespo-Hernández said. The team is also trying to identify which specific reactions in the cells decrease proliferation. Besides the ROS, energy and/or electron transfer reactions could also be playing important roles in the mechanism of light-induced cytotoxicity.
The research was funded by the National Science Foundation CAREER Program, grant CHE-1255084.
Story Source:
The above post is reprinted from materials provided by Case Western Reserve University.
The post An Expanded Genetic Code Is Shown Phototoxic to Cells appeared first on Scienmag.





