Tuberculosis, a persistent global health threat, impacts over 10 million individuals each year, and its prevalence has been notably pronounced in certain regions. The bacterium responsible for this condition, Mycobacterium tuberculosis, poses a multifaceted challenge to public health, as it spreads primarily through the air and infiltrates the lungs. The clinical presentation of tuberculosis includes a range of symptoms such as chronic cough, chest pain, fatigue, fever, and unintentional weight loss. Recent events, particularly a significant tuberculosis outbreak in Kansas, serve as stark reminders of the disease’s potential to cause severe morbidity and mortality, marking it as one of the largest outbreaks recorded in the United States.
Traditional interventions for tuberculosis primarily involve the utilization of antibiotics. However, the emergence of drug-resistant strains has further complicated treatment regimens and underscored the urgent necessity for novel drug candidates. In this context, exciting new research has surfaced, harnessing the power of artificial intelligence to identify potential antimicrobial compounds. This study, led by an interdisciplinary team comprising experts from the University of California San Diego, Linnaeus Bioscience Inc., and the Center for Global Infectious Disease Research at Seattle Children’s Research Institute, represents a groundbreaking step in tuberculosis research.
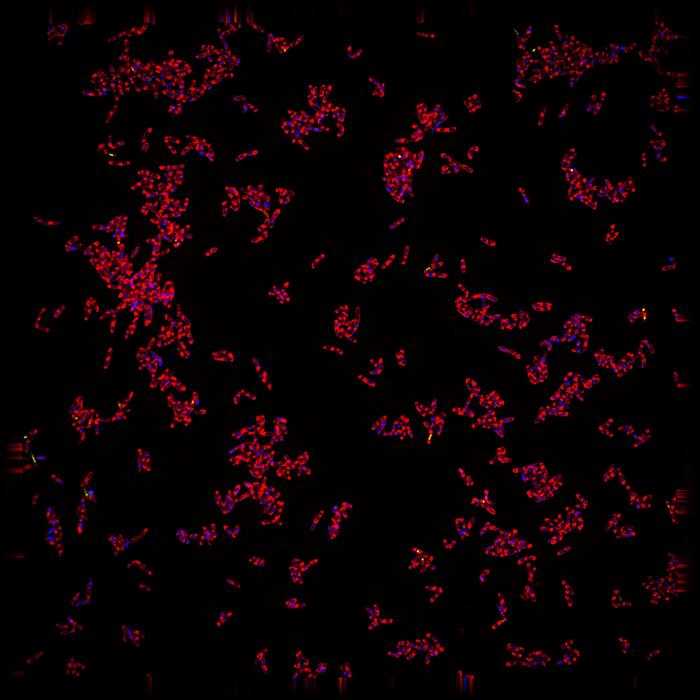
The research introduces a transformative technique known as MycoBCP, which integrates cutting-edge artificial intelligence with bacterial cytological profiling (BCP). This innovative method aims to revolutionize the understanding of how new therapeutics can effectively target Mycobacterium tuberculosis and illuminate the underlying mechanisms by which these antibiotics operate. BCP has long been an invaluable tool for comprehending bacterial responses to antibiotics, yet the application of deep learning in this domain is a pioneering leap forward that holds significant promise in addressing the challenges presented by tuberculosis.
The methodology involved in this research was meticulous and data-intensive, requiring the training of convolutional neural networks with an impressive dataset of over 46,000 images depicting tuberculosis cells. This comprehensive training enabled the AI technology to discern intricate patterns and variations that are often imperceptible to human observers. Joe Pogliano, a distinguished professor in the Department of Molecular Biology and a co-author of the study, articulated the revolutionary potential of the technology, emphasizing its capability to analyze bacterial images in a manner that transcends traditional lab techniques.
One of the primary challenges encountered in tuberculosis research is the difficulty of interpreting the visual data generated from microscopy imaging. Tuberculosis cells often appear clumped together, complicating efforts to delineate individual cell boundaries. Recognizing these constraints, the research team leveraged the strengths of advanced computer algorithms, allowing the machine to autonomously analyze the images for patterns indicative of antibiotic action. This automated approach represents a significant advancement in the field, rendering it easier to isolate candidates for further drug exploration.
Collaboration was a cornerstone of this project, bringing together experts from varied fields to enrich the research outcomes. Tanya Parish, a tuberculosis specialist at Seattle Children’s Research Institute, played a pivotal role in tailoring the BCP methodology specifically for mycobacterial species. The fusion of traditional bacterial profiling methods with artificial intelligence not only facilitated a streamlined research process but also significantly reduced the time required to identify promising compounds for drug development, thus expediting the journey from discovery to clinical application.
The implications of this research extend far beyond academic curiosity. With tuberculosis maintaining a stronghold as one of the deadliest infectious diseases globally, the need for rapid and effective treatment methodologies is paramount. The introduction of new candidates through this AI-driven approach not only fills the immediate gaps in antimicrobial treatment options but also enhances the capacity to prioritize drug development projects based on empirical data regarding their underlying mechanisms of action. The result is not merely an acceleration in research but a strategic alignment toward more effective disease management.
The establishment of Linnaeus Bioscience, rooted in the laboratories of UC San Diego in 2012, can be seen as a testament to the evolution of antibiotic research. With the BCP method emerging as a game-changer in how antibiotics are studied and understood, it has paved the way for new horizons in combating bacterial infections. The collaboration between academia and industry has proven essential; Linnaeus Bioscience has solidified its position as an influential entity in the biotechnology arena, providing vital services to partners and stakeholders worldwide.
As this research continues to unfold, the biotechnology community is poised for advancements that could radically alter the landscape of tuberculosis treatment. Joe Pogliano’s insights into the intersection of machine learning and traditional microbiology underscore a necessary evolution in the approach to infectious disease research. The collective commitment to harnessing innovative technology against enduring health crises is emblematic of a broader shift toward interdisciplinary methods that harness the latest scientific insights.
In conclusion, the successful integration of advanced AI methods into bacteriology represents not just a methodological advancement, but a potential paradigm shift in how researchers approach infectious diseases. By redefining the metrics of analysis and understanding the intricate behavior of Mycobacterium tuberculosis at the cellular level, this study heralds significant progress in the relentless pursuit of effective treatments for one of humanity’s most formidable public health adversaries.
With the continuous evolution of tuberculosis smart drug discovery, the scientific community remains vigilant. Their ongoing collaboration and commitment to innovation within Linnaeus Bioscience and beyond ensure that new analytical tools, such as MycoBCP, could bring crucial breakthroughs in addressing antibiotic resistance and enhancing therapeutic efficacy.
The prospect of emerging from the shadows of tuberculosis relies heavily on such inventive approaches and collaborative efforts, positioning researchers and biotechnology companies at the forefront of combating this acute global health challenge.
Subject of Research: Cells
Article Title: Deep learning–driven bacterial cytological profiling to determine antimicrobial mechanisms in Mycobacterium tuberculosis
News Publication Date: 7-Feb-2025
Web References: Proceedings of the National Academy of Sciences
References: None available, as this is a rewritten piece.
Image Credits: Linnaeus Bioscience
Keywords: Tuberculosis, Biotechnology, Drug candidates, Drug research, Bacterial infections, Artificial intelligence.
Tags: AI in tuberculosis researchantibiotic resistance in tuberculosisArtificial Intelligence in Medicinechronic cough and tuberculosis symptomsdrug discovery for tuberculosisglobal health threats of tuberculosisinnovative antimicrobial compounds developmentinterdisciplinary research in infectious diseasesMycobacterium tuberculosis challengesnovel drug candidates for TBpublic health strategies for tuberculosistuberculosis outbreak response