The global demand for iron and its alloys, primarily steel and cast iron, is consistently on the rise, driven by advancements in infrastructure, construction, and various industries. Traditionally, the process for extracting elemental iron from iron ore has relied heavily on blast furnaces, which are known for their high energy consumption and substantial environmental impact. However, a team of researchers have recently published a groundbreaking report in ACS Energy Letters, offering a compelling alternative that utilizes electrochemistry to produce iron more sustainably and cost-effectively from synthetic iron ores. This innovative methodology has the potential to change the ironmaking landscape dramatically, providing a pathway toward greener practices in metallurgy.
Historically, the smelting process in blast furnaces generates substantial greenhouse gas emissions and contributes to air pollution, primarily due to the combustion of fossil fuels. These emissions include not only carbon dioxide but also sulfur dioxide and fine particulate matter, which pose a serious risk to public health and the environment. As global awareness of climate change intensifies, the necessity for cleaner alternatives has never been more critical. The newly proposed electrochemical ironmaking process seeks to address these environmental shortcomings by leveraging electricity to extract iron from iron oxide without the need for high-temperature reduction.
Research spearheaded by Paul Kempler, who serves as the corresponding author of the study, highlights the significance of identifying iron oxide candidates capable of being reduced at lower temperatures. Lower temperature reductions are a key factor in developing a fully electrified and environmentally friendly ironmaking process that could rival conventional methods in terms of cost and efficiency. Electrochemical ironmaking involves a process whereby electrical currents are employed to reduce iron-containing feedstocks in an electrolytic cell, isolating the metal from its ores in a much cleaner fashion than traditional blast furnace technology allows.
In previous studies, Kempler and his team were able to transform solutions containing solid iron(III) oxide and sodium hydroxide into elemental iron at temperatures around 176 to 194 degrees Fahrenheit (80 to 90 degrees Celsius). Yet, when investigating the effectiveness of various natural iron ores with irregular particle sizes and impurities, the method yielded suboptimal results. Recognizing the challenges posed by the physical characteristics of these ores, Kempler subsequently collaborated with a team comprising Anastasiia Konovalova and Andrew Goldman to explore which iron ore-like feedstocks could enable scalable applications of their electrochemical methodology.
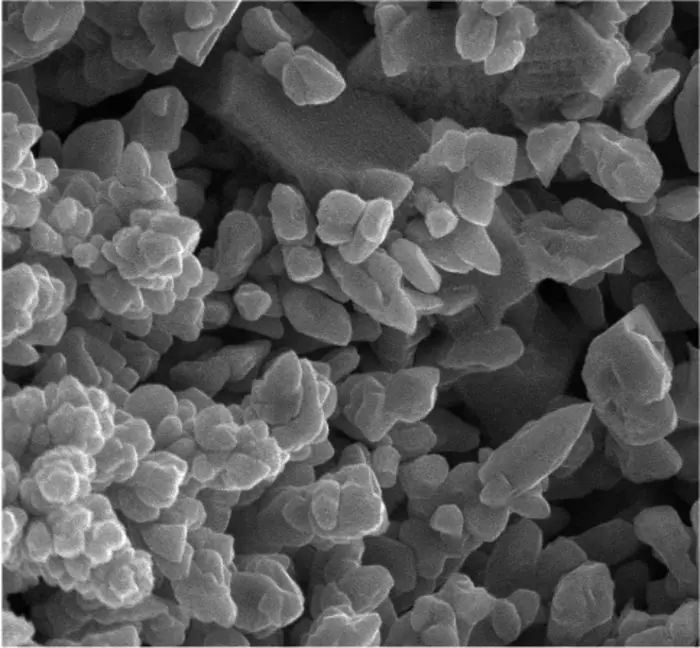
To further enhance the electrochemical reactions, the researchers prepared high surface area iron oxide particles that featured internal porosity and connected cavities. This nanoscale engineering of the particles was integral to assessing how varying morphologies influenced electrochemical performance. In a follow-up manipulation, the researchers synthesized micrometer-wide iron oxide particles that mirrored the natural structure of the ores sourced from the earth. These particles were carefully designed to contain minimal impurities, which included only trace amounts of carbon and barium.
During the experimentation phase, the team engineered a specialized cathode that allowed for the efficient extraction of iron metal from a sodium hydroxide solution containing the treated iron oxide particles. Remarkably, the dense iron oxides exhibited a selective reduction at a current density of 50 milliamperes per square centimeter—an efficiency comparable to the rapid charging rates observed in lithium-ion batteries. In contrast, particles exhibiting higher porosity demonstrated enhanced capabilities for effective electrochemical iron production, highlighting that morphological design is a pivotal variable affecting the overall success of the process.
As part of a comprehensive assessment, the researchers calculated the economic feasibility of their electrochemical ironmaking approach. They estimated production costs to be under $600 per metric ton (approximately $0.60 per kilogram), placing this technique on par with existing traditional ironmaking methods. Additionally, the study revealed the potential to achieve significantly higher current densities, approaching 600 milliamperes per square centimeter, akin to those utilized in contemporary industrial electrolysis cells. This indicates that advancements in both the design of electrochemical cells and methodologies for refining iron oxide feedstocks toward greater porosity are necessary catalysts for commercial viability.
The implications of this electrochemical approach extend beyond mere cost savings; the method offers substantial opportunities for reducing environmental impacts associated with conventional ironmaking. By minimizing carbon emissions and other harmful pollutants typically released in the course of traditional smelting processes, the electrochemical route marks a significant achievement in materials science and industrial production. The research team acknowledges the financial backing from the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, which was instrumental in bringing this innovative research to fruition.
While the study’s findings are groundbreaking, they are part of an evolving discourse on sustainable industrial practices. The insights gained could catalyze further research aimed at optimizing the electrochemical ironmaking process and unlocking its full potential. As more researchers engage in the effort to devise cleaner manufacturing processes, the hope is that industries across the globe will adopt greener technologies, significantly reducing their ecological footprints.
Furthermore, the research lays a solid foundation for future explorations into the electrochemical field, including the development of new materials, improved cell designs, and efficiencies of scale. The studies in electrochemical ironmaking offer a glimpse into a future where cleaner technologies not only coexist with but thrive alongside traditional methods, marking a new era of industrial production that prioritizes sustainability while meeting global demands.
In summary, the transition toward sustainable and efficient iron extraction through electrochemical methods encapsulates the necessity of innovation in the face of pressing environmental challenges. As the study associated with this novel approach continues to permeate the scientific community, it stands as a testament to the power of interdisciplinary collaboration in addressing some of the most formidable issues of our time. The journey toward mainstream adoption of these technologies is just beginning, but with continued research, the potential for transformative change remains within reach.
Subject of Research: Electrochemical ironmaking
Article Title: Pathways to Electrochemical Ironmaking at Scale Via the Direct Reduction of Fe2O3
News Publication Date: April 9, 2025
Web References: ACS Energy Letters DOI
References: ACS Energy Letters, DOI: 10.1021/acsenergylett.5c00166
Image Credits: Credit: Adapted from ACS Energy Letters 2025, DOI: 10.1021/acsenergylett.5c00166
Keywords
Iron, Electrochemical Process, Sustainable Manufacturing, Green Technology, Iron Oxide Reduction
Tags: advancements in sustainable metallurgyalternatives to traditional smeltingcleaner extraction methodscost-effective iron extraction solutionselectrochemical ironmaking processenvironmental benefits of electric iron productionimpact of blast furnacesimproving air quality through iron productioninnovative metallurgy techniquesreducing greenhouse gas emissionssustainable iron productionsynthetic iron ores