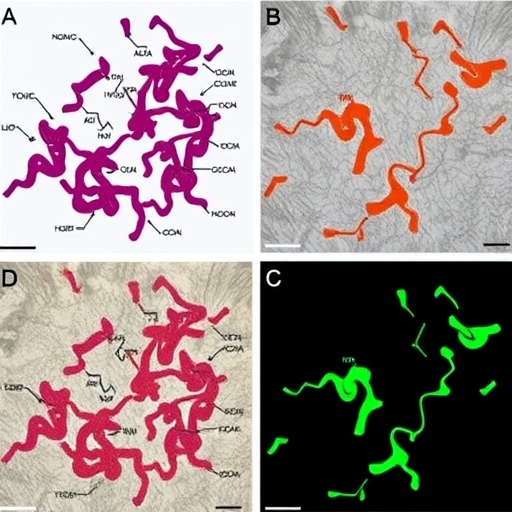
In a groundbreaking study published in the Journal of Medical Biology Engineering, researchers Chang, Hung, and Liu embark on an ambitious exploration into the realms of Alzheimerâs disease prediction and brain imaging. Utilizing state-of-the-art deep learning techniques, including U-Net and ResNet models, this research unveils advanced methods for hippocampus segmentation, a crucial component in understanding Alzheimerâs pathology. This study marks a significant leap forward in neuroimaging, offering potential for early detection and intervention in Alzheimerâs, a neurodegenerative disorder that affects millions worldwide.
Alzheimerâs disease presents a formidable challenge to public health, characterized by progressive cognitive decline and memory loss. Early diagnosis remains vital in configuring treatment approaches that could potentially slow disease progression. The hippocampus, a vital brain structure involved in memory formation and spatial navigation, is often one of the first areas to be affected by Alzheimerâs. The precise segmentation of the hippocampus through magnetic resonance imaging (MRI) is thus crucial to identify morphological changes indicative of Alzheimerâs disease.
The traditional methods of hippocampus segmentation often involve labor-intensive manual annotation, which can be subject to variability and human error. The research by Chang and colleagues propounds a solution by leveraging the power of deep learning architectures, primarily the U-Net and ResNet models, to automate the segmentation process. This automation not only enhances accuracy but also significantly reduces the time required for analysis, allowing clinicians and researchers to focus more on patient care and less on preprocessing data.
U-Net, a convolutional neural network architecture originally designed for biomedical image segmentation, plays a vital role in this study. The architecture employs a contracting path to capture context and a symmetric expanding path that enables precise localization. This dual function allows the U-Net to efficiently segment complex brain structures, such as the hippocampus, from MRI scans. The effectiveness of this architecture has been proven in various medical imaging tasks due to its ability to provide high-resolution outputs even from relatively small datasets.
On the other hand, the ResNet model, which incorporates residual learning to facilitate training of deeper networks, further complements the analysis. ResNet’s architecture ensures that the information from earlier layers in the network is preserved rather than lost in deeper layers. When applied to hippocampus segmentation, ResNet enhances performance by providing a robust framework for learning complicated representations, crucial for accurately identifying distinct features within the MRI images.
The methodology employed in this study involved the integration of U-Net and ResNet to exploit their individual strengths, thereby creating a hybrid model optimized for hippocampus segmentation. The researchers conducted extensive experimentation using a comprehensive dataset of brain MRI scans, ensuring a diverse range of images that reflect different stages of Alzheimerâs disease. This rigorous approach bolstered the generalizability of their findings, offering confidence that the model could perform effectively across varying circumstances.
In terms of performance metrics, the results obtained from this study underscore the potential of such deep learning architectures in clinical practice. The accuracy of the model was evaluated using standard metrics such as Dice Coefficient, Intersection over Union, and sensitivity, all of which demonstrated significant improvements over traditional segmentation methods. These quantitative findings provide compelling evidence for the deployment of machine learning techniques in enhancing diagnostic capabilities.
Moreover, the implications of this research extend beyond mere segmentation. The ability to accurately delineate the hippocampus in MRI scans can pave the way for developing predictive models that identify individuals at high risk for Alzheimerâs disease. With early detection, clinicians might craft tailored intervention strategies that could mitigate adverse outcomes. Therefore, the integration of advanced imaging techniques with machine learning not only addresses a technical challenge but also offers a hopeful path towards better disease management.
The study does not shy away from addressing the ethical considerations surrounding the use of deep learning in medical contexts. As algorithms increasingly assist in diagnostic processes, the importance of transparency in AI decision-making becomes paramount. The researchers emphasize the necessity for collaboration between data scientists and medical professionals to ensure that these advanced models align with clinical practices and uphold patient safety.
Looking to the future, the research lays a foundation for further studies aimed at refining these models and exploring additional features that could enhance prediction accuracy. The prospect of incorporating multi-modal data, such as genomic or neuropsychological information, into models arises, potentially offering a more comprehensive understanding of Alzheimerâs disease’s multifactorial nature.
Beyond the academic sphere, the practical applications of their findings could significantly influence healthcare infrastructure. As healthcare systems increasingly rely on technology, the integration of these machine learning techniques can streamline operations, reduce costs, and ultimately improve patient outcomes. This pioneering study illustrates how innovative approaches to data analysis have the ability to transform the landscape of neurodiagnostics.
In conclusion, Chang and colleaguesâ research stands as a testament to the power of combining advanced imaging technologies with deep learning models in the fight against Alzheimerâs disease. As the scope of artificial intelligence in healthcare expands, this study serves as a critical reminder that with innovation comes responsibility. Ensuring the efficacy and ethical deployment of these technologies is essential as they move from research settings into clinical practice.
With the rapid advancements in AI, the journey toward revolutionizing brain imaging and Alzheimerâs detection has only just begun. Further research will undoubtedly unlock even more potential, fueling hope for millions affected by this debilitating condition. Time will tell how soon these technological breakthroughs will translate into tangible benefits for patients suffering from Alzheimerâs disease and their families.
Subject of Research: Alzheimerâs Disease Prediction and Hippocampus Segmentation
Article Title: Enhanced Hippocampus Segmentation and Alzheimerâs Disease Prediction Using U-Net and ResNet Models on Brain Magnetic Resonance Imaging
Article References:
Chang, TA., Hung, CW. & Liu, XY. Enhanced Hippocampus Segmentation and Alzheimerâs Disease Prediction Using U-Net and ResNet Models on Brain Magnetic Resonance Imaging.
J. Med. Biol. Eng. (2025). https://doi.org/10.1007/s40846-025-00973-0
Image Credits: AI Generated
DOI:
Keywords: Alzheimerâs disease, U-Net, ResNet, hippocampus segmentation, deep learning, predictive modeling, neuroimaging
Tags: advanced brain imaging techniquesautomated segmentation in neuroimagingcognitive decline and memory lossdeep learning in medical imagingearly detection of Alzheimer’s diseasehippocampus morphology in AlzheimerâsMRI analysis for Alzheimer’s diagnosisneurodegenerative disorder prediction methodsneuroimaging advancements in dementiapublic health challenges in AlzheimerâsResNet application in Alzheimerâs researchU-Net model for hippocampus segmentation