Credit: Les Laboratoires Servier, courtesy of Servier Medical Art (), reproduced under the Creative Commons Attribution 3.0 France (CC BY 3.0 FR) license (https://creativecommons.org/licenses/by/3.0/fr/)…
An international research team in which the DZD is participating has identified a novel adipokine that favors the development of insulin resistance and systemic inflammation. In cases of severe obesity, this adipokine is secreted by the adipocytes of the abdominal fat tissue and released into the bloodstream. The new findings could contribute to the development of alternative approaches for the treatment of diseases caused by obesity. The researchers have now published their results in the journal Diabetologia (Hörbelt et al, 2018) (1) of the European Association for the Study of Diabetes (EASD).
More than 2.8 million people die each year due to conditions related to overweight and obesity (2). Overweight and the associated metabolic syndrome (3) increase the risk of type 2 diabetes, specific types of cancer and cardiovascular disease. Scientific findings in recent years have confirmed this increased risk. The cause of the sequelae are chronic inflammatory responses. However, the molecular mechanisms that lead to these overweight-related inflammatory processes are still largely unknown. This is the starting point for the study of the international team of scientists led by PD Dr. Natalia Rudovich (Spital Bülach; Charité – Universitätsmedizin Berlin), Prof. Dr. Margriet Ouwens (German Diabetes Center Düsseldorf) and PD Dr. Olga Pivovarova of the German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE).
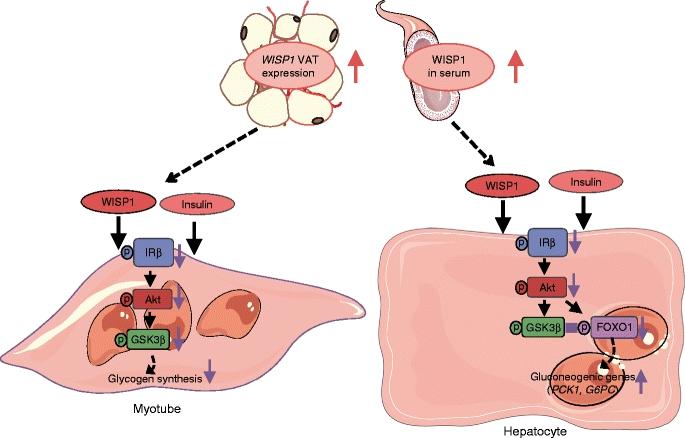
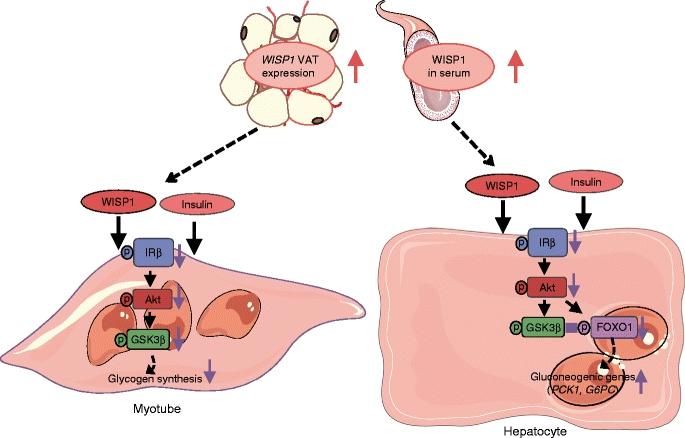
The researchers showed for the first time how the protein molecule Wingless-type signaling pathway protein-1 (WISP1) directly impairs insulin action in muscle cells and in the liver and thus leads to reduced insulin sensitivity. Already in 2015, the team led by the physician Rudovich and the biologist Pivovarova identified WISP1 as another possible link between obesity and systemic inflammatory responses (4). WISP1 was previously associated with the regulation of bone growth, the development of certain types of cancers and pulmonary fibrosis.
The current study shows that WISP1 cancels insulin-induced inhibition of glucose production (gluconeogesis) (5) in murine hepatocytes and glycogen synthesis (6) in human muscle cells. The synthesis quantity of the WISP1 protein correlates with the blood glucose levels in the oral glucose tolerance test (OGTT) and with the circulating level of heme oxygenase-1 (HO-1), an enzyme that promotes systemic inflammation, especially in obesity (7). "We suspect that increased WISP1 production from abdominal fat could be one of the reasons why overweight people often have an impaired glucose metabolism," said first author Tina Hörbelt of the German Diabetes Center Düsseldorf, a partner of the DZD. "One possible cause of increased WISP1 production and secretion from the abdominal fat cells could be the poor oxygen supply (hypoxia) of the tissues. This could lead to systemic inflammatory responses," explained DIfE researcher Pivovarova.
The new findings open up alternative approaches to the treatment of diseases caused by obesity. "For example, novel drugs could target and specifically prevent the WISP1 effect on muscles and liver cells and thus lead to improved insulin action in these tissues," said Rudovich, head diabetologist and endocrinologist at Spital Bülach. "However, it is still a long way from basic research (8) to a viable treatment", the physician added. Nevertheless, the new findings would already contribute to a better understanding of the relationships between obesity, the immune system and metabolic diseases.
###
Background Information
1The novel adipokine WISP1 associates with insulin resistance and impairs insulin action in myotubes and hepatocytes. Hörbelt, T., Tacke, C., Markova, M. et al. Diabetologia (2018). https://doi.org/10.1007/s00125-018-4636-9
2WHO Report 2017
3The metabolic syndrome is a combination of obesity, hypertension, insulin resistance of the body's cells and a disturbed lipid metabolism.
4WISP1 is a novel adipokine linked to inflammation in obesity. Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani-Nejad F, Gögebakan Ö, Osterhoff M, Kemper M, Hornemann S, Markova M, Klöting N, Stockmann M, Weickert MO, Lamounier-Zepter V, Neuhaus P, Konradi A, Dooley S, von Loeffelholz C, Blüher M, Pfeiffer AF, Rudovich N. Diabetes. 2015 Mar;64(3):856-66. doi: 10.2337/db14-0444.
5Gluconeogenesis is a metabolic pathway for the synthesis of glucose from non-carbohydrates and serves to maintain a constant blood glucose level even during periods of fasting.
6 Glycogen synthesis serves the organism to produce the carbohydrate storage form glycogen from glucose.
7 Jais, A. et al.: Heme Oxygenase-1 Drives Metaflammation and Insulin Resistance in Mouse and Man. Cell 2014, 158(1), 25-40. http://doi.org/10.1016/j.cell.2014.04.043
8The published data are part of the study "Unravelling the role of WISP1 on metabolic and cellular plasticity in white adipose Tissue" (Natalia Rudovich and Margriet Ouwens), funded by the European Foundation for Study of Diabetes (EFSD), and the project "WISP1 is a novel target for regulation of glucose metabolism" (Natalia Rudovich and Margriet Ouwens), funded by the German Diabetes Center Düsseldorf
The German Center for Diabetes Research (DZD) is one of six German Centers of Health Research. It brings together experts in the field of diabetes research and combines basic research, epidemiology and clinical applications. By adopting an innovative, integrative approach to research, the DZD aims to make a substantial contribution to the successful, personalized prevention, diagnosis and treatment of diabetes mellitus. The members of the association are Helmholtz Zentrum München – German Research Center for Environmental Health, the German Diabetes Center Düsseldorf (DDZ), the German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), the Institute of Diabetes Research and Metabolic Diseases of Helmholtz Zentrum München at the University of Tübingen, and the Paul Langerhans Institute Dresden of Helmholtz Zentrum München at the University Medical Center Carl Gustav Carus of TU Dresden, associated partners at the universities in Heidelberg, Cologne, Leipzig, Lübeck and Munich, and other project partners.
With 200 beds and around 1,100 employees, Spital Bülach provides nearby, high-quality medical care for the population in the Zurich Unterland. The Internal Medicine Clinic of Spital Bülach has been certified as A-Clinic by the Swiss Institute for Continuing Medical Education (SIWF). In internal medicine, it fulfills the same high criteria in further education as Zurich University Hospital, Winterthur Cantonal Hospital or Spital Uster.
DIfE is a member of the Leibniz Association. It investigates the causes of nutrition-related diseases in order to develop new strategies for prevention, treatment and nutritional recommendations. Its research focuses on obesity, diabetes, cardiovascular diseases and cancer. DIfE is also a partner of the German Center for Diabetes Research (DZD), funded by the BMBF since 2009.
Contact:
PD Dr. Natalia Rudovich
Head Physician Endocrinology/Diabetology
Spital Bülach
Spitalstrasse 24, 8180-Bülach/Switzerland
phone: +41-44863-25-30
e-mail: [email protected]
and
Charité – Universitätsmedizin Berlin
Medizinische Klinik für Endokrinologie und Stoffwechselmedizin
Campus Benjamin Franklin
Hindenburgdamm 30
12200 Berlin
PD. Dr. Olga Pivovarova
Department of Clinical Nutrition
German Institute of Human Nutrition
Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49-0-33200-88-2771
e-mail: [email protected]
Media Contact
Birgit Niesing
[email protected]
49-893-187-3971
@diabresearch
http://www.dzd-ev.de
Original Source
https://www.dzd-ev.de/en/press/press-releases/press-releases-2018/abdominal-fat-secretes-novel-adipokine-promoting-insulin-resistance-and-inflammation/index.html http://dx.doi.org/10.1007/s00125-018-4636-9