Arecent study by UPF’s BCN MedTech Research Unit has outlined a new computational model that simulates the interactions between the proteins present in joint cells and helps pinpoint the factors that cause osteoarthritis in each patient. The development of this computational model is particularly relevant, given the fact that the factors which produce this disease may be different for each person. Therefore, identifying the specific causes in each case is key to treating osteoarthritis in a more personalised and efficient manner.
Credit: Authors of the research
Arecent study by UPF’s BCN MedTech Research Unit has outlined a new computational model that simulates the interactions between the proteins present in joint cells and helps pinpoint the factors that cause osteoarthritis in each patient. The development of this computational model is particularly relevant, given the fact that the factors which produce this disease may be different for each person. Therefore, identifying the specific causes in each case is key to treating osteoarthritis in a more personalised and efficient manner.
This research was divulged in the article “Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis”, which was published on 3 February in the journal Frontiers in Bioengineering and Biotechnology and written by three researchers from the UPF Department of Information and Communication Technologies’ (DTIC) Barcelona Centre for New Medical Technologies (BCN MedTech). The researchers are Maria Segarra-Queralt, the article’s main author and member of BCN MedTech; Gemma Piella, head of this Research Unit and full DTIC professor; and Jérôme Noailly, coordinator of the Biomechanics and Mechanobiology Area of BCN MedTech and the study’s principal investigator.
Close to 7 million people suffer from osteoarthritis in Spain
The article focuses on osteoarthritis, a disease stemming from the loss of cartilage in joints, the tissue that covers the ends of long bones and which ensures that the joints function properly and absorbs any shocks they may receive over the course of their life. The most common and debilitating form of this disease is osteoarthritis of the knee. Patients who suffer from this disease usually experience intense pain in the affected joints and a loss of mobility. As a result, people with osteoarthritis often suffer chronic pain, depression or insomnia, symptoms which, due to their discomfort, render the individuals even more sedentary, which in turn exacerbates their condition or other chronic diseases.
The incidence of osteoarthritis tends to be higher in Western countries due to the ageing population, as its prevalence increases with age, and the more sedentary lifestyles (which are both a cause and consequence of the disease). According to data from the Spanish Society for Family and Community Medicine, courtesy of AECOSAR (the Spanish Association for Osteoporosis and Osteoarthritis), in Spain, this illness affects close to 7 million people, with the subsequent burden this places on the social, economic and healthcare system.
Jérôme Noailly, the study’s principal investigator: “With today’s technologies, it is extremely complex for doctors to determine the causes of the osteoarthritis in each patient and choose the most appropriate course of treatment”
At present, osteoarthritis is treated solely with palliative therapies such as exercise, analgesics or prosthetic surgery (knee, hip…). Until now, effective therapies for halting its progression or regenerating degraded cartilage have been particularly difficult to find given the multifactorial origin of the disease. While its origin has traditionally been linked to mechanical factors such as trauma or excess stress, it may also be the result of pro-inflammatory or genetic factors. The inflammation-related causes of osteoarthritis are primarily linked to ageing, yet also to the coexistence of other diseases, and affect women and men differently (there is a higher incidence among women). Certain types of diseases (e.g. metabolic diseases) increase the risk of inflammatory blood cells infiltrating joints, making the person more likely to experience osteoarthritis. Pro-inflammatory factors, which also appear in the joint, degrade the cartilage and aggravate the pain, and may lead to central nervous system sensitisation, a series of conditions which prove difficult to control. In short, there are numerous factors that make certain people more prone than others to developing osteoarthritis, and the pain is different for each person. Therefore, the therapeutic strategies deployed in each case must also be different.
In this regard, according to Jérôme Noailly, the study’s principal investigator: “With today’s technologies, it is extremely complex for doctors to determine the causes of the osteoarthritis and pain in each patient and choose the most appropriate course of treatment.” Noailly also adds that: “Until now, it has been difficult for doctors to distinguish whether the disease’s main symptom, such as chronic pain, is of neurogenic or inflammatory origin.” Neither has it been possible to define the mechanical profile of each patient, i.e. the impact of mechanical movements (excessive stress, torsion…) on the cells in articular cartilage. Determining each patient’s mechanical profile is also key to tailoring the therapies to each person’s individual needs, as the reaction of the cartilage cells to biochemical stimuli depends on the mechanical loads.
Maria Segarra, the study’s main author: “We believe that our model is a step towards personalised medicine, which is key to addressing multifactorial diseases such as osteoarthritis”
In efforts to make headway in the pursuit of more effective and personalised treatments and medications for osteoarthritis, the researchers from UPF’s BCN MedTech have devised a model that involves using fast scanning to rapidly assess the biological and mechanical profile of each patient and the most efficient type of therapy. “We could obtain a picture of each patient’s biological profile, which would enhance our clinical diagnostic capabilities. We therefore believe that our model is a step towards personalised medicine, which is key to addressing multifactorial diseases such as osteoarthritis,” explains Maria Segarra, the study’s main author.
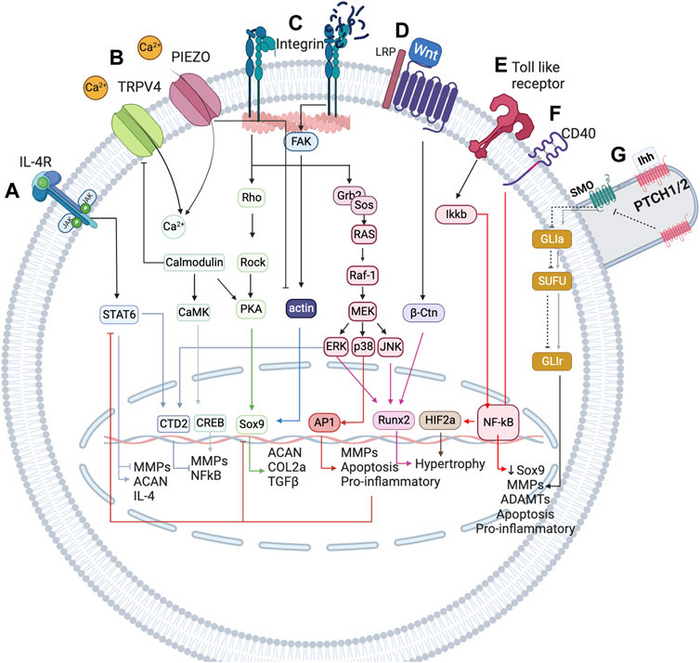
This model is defined by examining the impact of mechanical and inflammatory stimuli on articular cartilage cells (or chondrocytes). To link the state of these cells with mechanical and inflammatory factors, the researchers developed a network-based computational model that integrates a wealth of knowledge on the biology of articular cartilage to simulate mechano-biological behaviour. The set of 118 nodes from the network represent proteins and other important molecules in chondrocyte regulation, as well as the 300 interactions between the molecules.
Using this computational model, the researchers analysed the behaviour of the chondrocytes in four cases: under homoeostatic conditions, which represent healthy or safe situations; under pro-inflammatory conditions; in adverse mechanical situations (static postures, compression, torsion…); and following the application of regenerative anti-inflammatory therapy. They also conducted a sensitivity analysis to determine which of these situations had the biggest effect on chondrocyte metabolism, be it positive or negative.
The study’s main findings on the causes of osteoarthritis
The study made four significant findings regarding the overall causes for the development or aggravation of osteoarthritis. Firstly, the researchers concluded that pro-inflammatory molecules (the so-called pro-inflammatory cytokines) have the greatest influence on the development and progression of the condition. Secondly, the research verified that sedentary behaviour (or other situations in which the cartilage is subject to low-intensity yet static and persistent mechanical loads) does indeed aggravate the inflammatory pain caused by osteoarthritis. Thirdly, it determined that osteoarthritis treatments based on anti-inflammatory molecules from the patient themself may help decrease inflammatory pain signals. Fourthly, the study shows that mechanical loads (e.g. being overweight) inhibit the cartilage’s ability to regenerate.
This research was conducted with support and financing from the Government of Catalonia and the Agency for the Management of University and Research Grants (grant no. 2020 FI B 00680), the Spanish Ministry of Science and Innovation (MICINN) and the Spanish Research Agency (AEI) through the STRATO knowledge generation project (PID2021-126469OB-C21), as well as the European Commission and European Research Council (ERC) through the Disc4All (MSCA- 2020-ITN-ETN GA: 955735) and O-Health (ERC-2021-CoG-101044828) projects.
Reference article:
“Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis”
Maria Segarra-Queralt, Gemma Piella and Jérôme Noailly
Frontiers in Bioengineering and Biotechnology, 3 February 2023
DOI: https://doi.org/10.3389/fbioe.2023.1006066
Journal
Frontiers in Bioengineering and Biotechnology
DOI
10.3389/fbioe.2023.1006066
Method of Research
Computational simulation/modeling
Subject of Research
People
Article Title
Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis
Article Publication Date
3-Feb-2023



