Chirality of molecules is a crucial consideration in drug development as the enantiomeric purity of a compound can significantly impact its pharmacological properties. Chiral molecules possess mirror-image enantiomers that can exhibit distinct pharmacological properties from each other. Techniques for the synthesis and separation of pure and useful enantiomers with desirable properties are thus widely desired. Furthermore, techniques like high-performance liquid chromatography (HPLC) that enable the recycling of samples are also being used for enhancing the efficiency of separation. Additionally, methods for enhancing the functionality of the recycling system and increasing the efficiency of separation are being increasingly studied.
Credit: Hideyo Takahashi and Kumi Tozawa from Tokyo University of Science
Chirality of molecules is a crucial consideration in drug development as the enantiomeric purity of a compound can significantly impact its pharmacological properties. Chiral molecules possess mirror-image enantiomers that can exhibit distinct pharmacological properties from each other. Techniques for the synthesis and separation of pure and useful enantiomers with desirable properties are thus widely desired. Furthermore, techniques like high-performance liquid chromatography (HPLC) that enable the recycling of samples are also being used for enhancing the efficiency of separation. Additionally, methods for enhancing the functionality of the recycling system and increasing the efficiency of separation are being increasingly studied.
To transform an undesirable enantiomer into a desirable one, a process known as “racemization” can be employed. This method involves converting a chiral molecule into a racemic mixture, which is a combination of both enantiomers in equal proportions. The desired enantiomer can then be isolated from the mixture through various separation and purification methods. A team of scientists from Japan have previously made significant progress in the rapid racemization of chiral sulfoxides, which are important bioactive molecules used as antiulcer drugs. They achieved this by using 2,4,6-triphenylpyrylium tetrafluoroborate (TPT+) as a photocatalyst to catalyze the reaction under mild conditions.
Furthering their research, the team, led by Professor Hideyo Takahashi along with co-author Kumi Tozawa from Tokyo University of Science in Japan, have now developed a recycling photoreactor that allows the production of enantiomerically pure chiral sulfoxides from a racemic mixture. This breakthrough development, consisting of a two-step rapid photoracemization of chiral sulfoxides on a solid phase, could have significant implications for the pharmaceutical drug development. This study was published in the Journal of Organic Chemistry on May 08, 2023.
“A recycle photoreactor utilizing the concept of deracemization, where a racemate is converted into a pure enantiomer, is developed and successfully applied in the syntheses of chiral alkyl aryl sulfoxides,” says Prof. Takahashi.
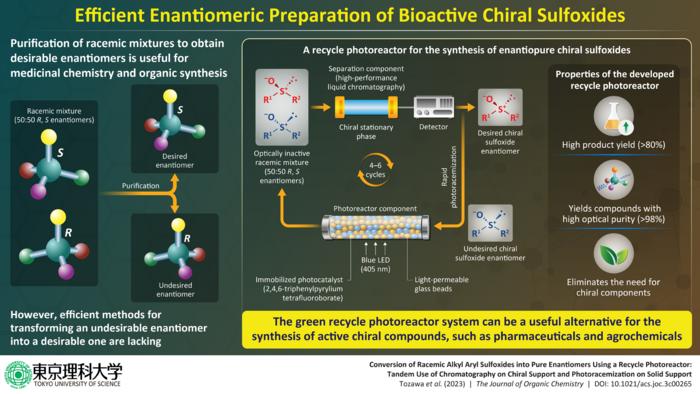
The developed recycle photoreactor is composed of a photoreaction section and a separation section where the racemization and separation occur respectively. The photoreaction section comprises a glass column containing the photocatalyst 2,4,6-triphenylpyrylium tetrafluoroborate (TPT+) immobilized on a resin, while the separation section consists of an HPLC column containing a chiral stationary phase that selectively interacts with and separates the desired enantiomer from the racemic mixture.
To obtain the desired enantiomer, the undesired enantiomer is initially introduced into the photoreaction section, where it undergoes photoracemization upon irradiation with light from a blue LED. Subsequently, the resulting racemic mixture is passed through the HPLC column, which separates the desired enantiomer. The undesired enantiomer is recycled back to the photoreaction section, and the process is repeated until a high amount of the enantiomerically pure compound is obtained.
Using the developed system, the team of researchers successfully synthesized four enantiomerically pure chiral alkyl aryl sulfoxides, achieving a high optical purity of 98–99%. These sulfoxides, namely 2-methoxyethyl phenyl sulfoxide, cyclopropyl phenyl sulfoxide, and methoxyphenyl methyl sulfoxide, were obtained in yields higher than 80% following four to six cycles.
The proposed photoreactor system thus allows for an efficient and scalable production of enantiomerically pure compounds without the need for chiral components, making it a sustainable technique for the synthesis of low-molecular-weight compounds, such as pharmaceuticals and agrochemicals. “If these can be manufactured at low cost, it will lead to a stable supply and lower prices for pharmaceuticals and other products,” says Prof. Takahashi, speaking of the possible real-world implications of their achievement.
We wish the team luck in their ongoing efforts, which will undoubtedly find attractive applications in pharmaceutical manufacturing.
***
Reference
DOI: https://doi.org/10.1021/acs.joc.3c00265
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Hideyo Takahashi from Tokyo University of Science
Hideyo Takahashi is a Professor at the Faculty of Pharmaceutical Sciences, Tokyo University of Science, Japan. She earned her undergraduate degree from The University of Tokyo in 1989 and PhD degree from the Tokyo University of Science in 1994. Her research areas cover medicinal chemistry, catalytic enantioselective asymmetric synthesis, and carbohydrate chemistry. Her present field of work includes axial chirality. She has received the prestigious PSJ Award for Young Scientists for her contribution to the field. Professor Takahashi is affiliated with the Pharmaceutical Society of Japan and the Society of Synthetic Organic Chemistry, Japan.
Funding information
This study was partially supported by A-STEP (JPMJTR213B) from the Japan Science and Technology Agency.
Journal
The Journal of Organic Chemistry
DOI
10.1021/acs.joc.3c00265
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Conversion of Racemic Alkyl Aryl Sulfoxides into Pure Enantiomers Using a Recycle Photoreactor: Tandem Use of Chromatography on Chiral Support and Photoracemization on Solid Support
Article Publication Date
8-May-2023
COI Statement
The authors declare no competing financial interest




