Metals are typically used as active materials for negative electrodes in batteries. Recently, redox-active organic molecules, such as quinone- and amine-based molecules, have been used as negative electrodes in rechargeable metal–air batteries with oxygen-reducing positive electrodes. Here, protons and hydroxide ions participate in the redox reactions. Such batteries exhibit high performance, close to the maximum capacity that is theoretically possible. Furthermore, using redox-active organic molecules in rechargeable air batteries overcomes problems associated with metals, including the formation of structures called ‘dendrites,’ which impact battery performance, and have negative environmental impact. However, these batteries use liquid electrolytes—just like metal-based batteries—which pose major safety concerns like high electrical resistance, leaching effects, and flammability.
Credit: Kenji Miyatake from Waseda University
Metals are typically used as active materials for negative electrodes in batteries. Recently, redox-active organic molecules, such as quinone- and amine-based molecules, have been used as negative electrodes in rechargeable metal–air batteries with oxygen-reducing positive electrodes. Here, protons and hydroxide ions participate in the redox reactions. Such batteries exhibit high performance, close to the maximum capacity that is theoretically possible. Furthermore, using redox-active organic molecules in rechargeable air batteries overcomes problems associated with metals, including the formation of structures called ‘dendrites,’ which impact battery performance, and have negative environmental impact. However, these batteries use liquid electrolytes—just like metal-based batteries—which pose major safety concerns like high electrical resistance, leaching effects, and flammability.
Now, in a new study published in Angewandte Chemie International Edition on May 2, 2023, a group of Japanese researchers have developed an all-solid-state rechargeable air battery (SSAB) and investigated its capacity and durability. The study was led by Professor Kenji Miyatake from Waseda University and the University of Yamanashi, and co-authored by Professor Kenichi Oyaizu from Waseda University.
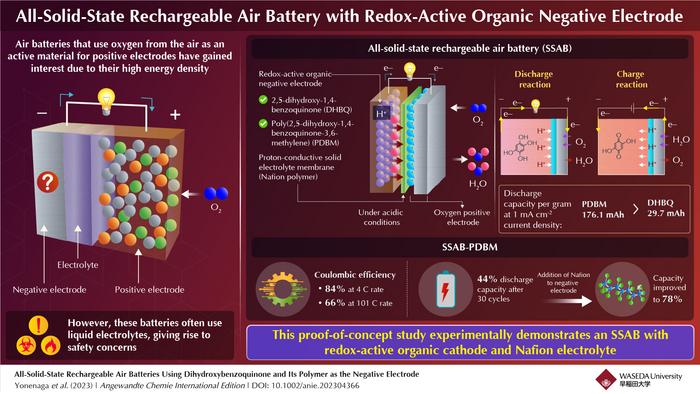
The researchers chose a chemical called 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) (PDBM) as active materials for the negative electrode due to their stable and reversible redox reactions in acidic conditions. In addition, they utilized a proton-conductive polymer called Nafion as the solid electrolyte, thereby replacing conventional liquid electrolytes. “To the best of my knowledge, no air batteries based on organic electrodes and solid polymer electrolyte have been developed yet,” says Miyatake.
After the SSAB was in place, the researchers experimentally assessed its charge–discharge performance, rate characteristics, and cyclability. They found that unlike typical air batteries that use a metallic negative electrode and an organic liquid electrolyte, the SSAB did not deteriorate in the presence of water and oxygen. Furthermore, replacing the redox-active molecule DHBQ with its polymeric counterpart PDBM formed a better negative electrode. While the per gram-discharge capacity of the SSAB-DHBQ was 29.7 mAh, the corresponding value of the SSAB-PDBM was 176.1 mAh, at a constant current density of 1 mAcm-2.
The researchers also found that the coulombic efficiency of SSAB-PDBM was 84% at 4 C rate, which gradually decreased to 66% at 101 C rate. While the discharge capacity of SSAB-PDBM reduced to 44% after 30 cycles, by increasing the proton-conductive polymer content of the negative electrode, the researchers could significantly improve it to 78%. Electron microscopic images confirmed that the addition of Nafion improved the performance and durability of the PDBM-based electrode.
This study demonstrates the successful operation of an SSAB comprising redox-active organic molecules as the negative electrode, a proton-conductive polymer as the solid electrolyte, and an oxygen-reducing, diffusion type positive electrode. The researchers hope that it will pave the way for further advancements. “This technology can extend the battery life of small electronic gadgets such as smartphones and eventually contribute to realizing a carbon-free society,” concludes Miyatake.
***
Reference
DOI: https://doi.org/10.1002/anie.202304366
Authors: Makoto Yonenaga1, Yusuke Kaiwa2, Kouki Oka2,3, Kenichi Oyaizu2, and Kenji Miyatake1
Affiliations:
1Clean Energy research Center, Fuel Cell Nanomaterials Center, University of Yamanashi
2Department of Applied Chemistry, Research Institute for Science and Engineering, Waseda University
3Center for Future Innovation (CFI) and Department of Applied Chemistry, Graduate School of Engineering, Osaka University
Funding Information
This work was partly supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, through Grants-in-Aid for Scientific Research (18H05515, 23H02058), MEXT Program: Data Creation and Utilization Type Material Research and Development Project (JPMXP1122712807), and JKA promotion funds from AUTORACE.
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
About Professor Kenji Miyatake
Kenji Miyatake received his Ph.D. degree in chemistry from Waseda University in 1996. He was a Japan Society for the Promotion of Science (JSPS) postdoctoral fellow at McGill University from 1999 to 2001. In 2001, he was offered an associate professor position at the Clean Energy Research Center at the University of Yamanashi, where he currently serves as a professor. He also holds a professor position in his alma mater since 2020. He is also a Fellow of the Royal Society of Chemistry.
DOI
10.1002/anie.202304366
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
All-Solid-State Rechargeable Air Batteries Using Dihydroxybenzoquinone and Its Polymer as the Negative Electrode
Article Publication Date
2-May-2023
COI Statement
The authors declare no conflict of interest




