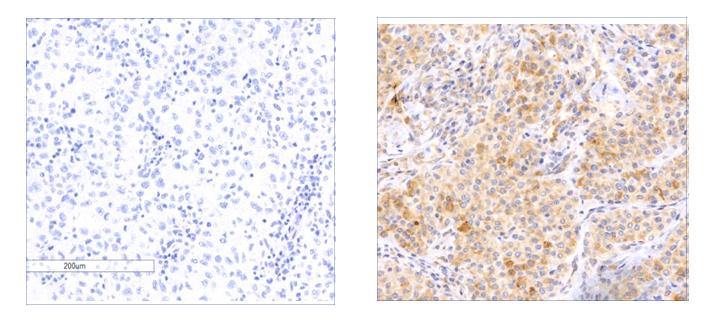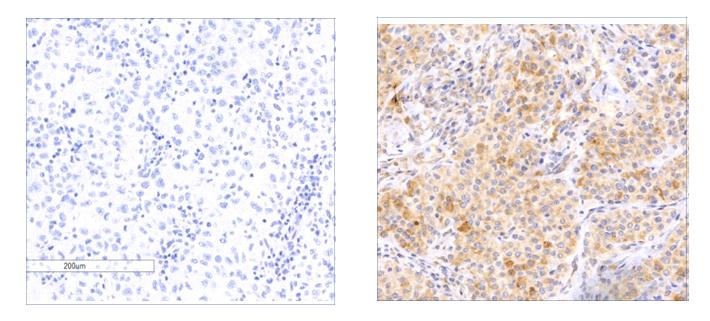
Of the hundreds of genes that can be mutated in a single case of melanoma, only a handful may be true "drivers" of cancer. In research that appeared today in Nature Genetics, a Weizmann Institute of Science team has now revealed one of the drivers of a particularly deadly subset of melanomas – one that is still seeing a rise in new cases. This gene is a newly identified member of a group of genes called tumor suppressor genes. It is mutated in some 5.4% of melanomas. Furthermore, its expression was found to be lost in over 30% of human melanomas; and this loss, according to the finding, was associated with reduced patient survival. This discovery might open new doors to understanding how this cancer grows and spreads, and it may lead in the future to new directions in treating this disease.
Prof. Yardena Samuels and her team in the Institute's Molecular Cell Biology Department were specifically searching for tumor suppressor genes in their database, which consists of more than 500 melanoma genomes and exomes – protein-building sequences – making it the largest melanoma dataset to date. As their name suggests, tumor suppressor genes normally inhibit cell growth, including that of cancer cells. However, when mutated, they act like defective brakes on cellular proliferation. Thus studying these genes is crucial in cancer biology. "The identification of targetable alterations in melanoma is an urgent need. An in-depth understanding of the functional effects of mutations in these genes is the first step toward revealing the underlying mechanism of melanoma growth," says Dr. Nouar Qutob, a postdoctoral fellow in Samuels' lab who participated in this research.
Indeed, the melanoma genome sequences contained mutations in known tumor suppressor genes, but there was also a new gene that stood out in the team's search, named RASA2. The researchers' next step was to conduct a series of functional experiments to understand exactly what this gene does. They cloned both the normal protein and the most recurrent mutated versions to see their effects on melanoma cells. They found that RASA2 regulates a key protein in the cell, called RAS. RAS has been identified as a major oncogene that contributes to the unchecked growth of cells. When they restored the production of the protein in melanoma cells that harbored RASA2 mutations, these cells stopped growing and eventually died.
Patients with dysfunctional RAS pathways tend to have a worse prognosis than those with other types of melanoma, and, until now, scientists have not managed to create drugs that can target this pathway. "As the RAS pathway is highly dysregulated in cancer, the discovery of an alternative mechanism for its activation is likely to stimulate an avalanche of further research in this field, and is highly likely to have direct clinical relevance. We are now going to focus on RASA2, to find out what proteins it communicates with in healthy cells and melanoma, as well as in the cells' response to targeted therapy," says Samuels. "Most targeted cancer therapies nowadays work by inhibiting the products of oncogenes that are overactive in melanoma cells. However, loss or mutations in tumor suppressor genes like RASA2 also contribute to melanoma development; therefore, discovering and studying RASA2 targets and partners will be our next aim," says Rand Arafeh, a PhD student in Samuels' lab and lead author of the paper.
###
Prof. Yardena Samuels' research is supported by the Ekard Institute for Diagnosis, which she heads; the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics; the Laboratory in the name of M.E.H Fund established by Margot and Ernst Hamburger; the Louis and Fannie Tolz Collaborative Research Project; the Dukler Fund for Cancer Research; the European Research Council; the De Benedetti Foundation-Cherasco 1547; the Peter and Patricia Gruber Awards; the Comisaroff Family Trust; the Rising Tide Foundation; Sharon Zuckerman, Canada; Charles Rothschild, Brazil; the estate of Alice Schwarz-Gardos; the estate of John Hunter; and the estate of Adrian Finer. Prof. Samuels is the incumbent of the Knell Family Professorial Chair.
The Weizmann Institute of Science in Rehovot, Israel, is one of the world's top-ranking multidisciplinary research institutions. Noted for its wide-ranging exploration of the natural and exact sciences, the Institute is home to scientists, students, technicians and supporting staff. Institute research efforts include the search for new ways of fighting disease and hunger, examining leading questions in mathematics and computer science, probing the physics of matter and the universe, creating novel materials and developing new strategies for protecting the environment.