Aromatic ketones have long been valuable intermediates in chemical synthesis, particularly in cross-coupling reactions where different chemical entities are combined to form new compounds. For instance, a process called deacylative cross-coupling removes the acyl group from the aromatic ketone, allowing it to bond with other chemicals and produce a wide variety of useful compounds. These reactions are crucial for producing a wide array of aromatic compounds used in various industries like agrochemicals.
Aromatic ketones have long been valuable intermediates in chemical synthesis, particularly in cross-coupling reactions where different chemical entities are combined to form new compounds. For instance, a process called deacylative cross-coupling removes the acyl group from the aromatic ketone, allowing it to bond with other chemicals and produce a wide variety of useful compounds. These reactions are crucial for producing a wide array of aromatic compounds used in various industries like agrochemicals.
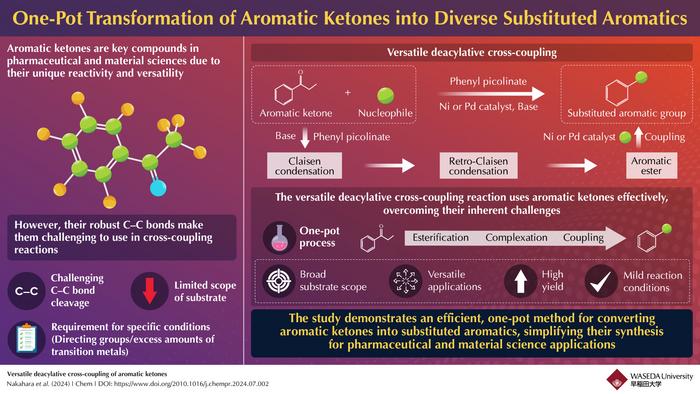
However, the utility of aromatic ketones has been limited due to the difficulty in breaking their strong carbon-carbon bonds. These robust bonds are challenging to cleave, often requiring specific conditions or catalysts. Traditional methods for overcoming this challenge have involved complex and costly procedures, which include the use of directing groups and large amounts of transition metals. These limitations can complicate the process and increase overall costs, hindering the broader application of aromatic ketones in various industrial and synthetic contexts.
To address traditional challenges in using aromatic ketones, a team of researchers led by Professor Junichiro Yamaguchi from the Faculty of Science and Engineering, School of Advanced Science and Engineering at Waseda University has developed a groundbreaking one-pot method that optimizes the conversion of aromatic ketones into aromatic esters. The research, published in Chem on 12 September 2024, highlights its potential for broader use in synthetic chemistry.
This new approach significantly simplifies the reaction process by integrating a Claisen reaction and a retro-Claisen reaction into a single step. The Claisen reaction combines two molecules to form a larger intermediate compound, while the retro-Claisen reaction modifies this intermediate to produce the desired ester. By combining these steps into a one-pot process, the researchers have managed to simplify the synthesis, reduce reaction times, and minimize the need for additional purification steps. “This advancement enhances the versatility and utility of aromatic ketones, facilitating their use in pharmaceutical synthesis and materials science,” remarks Dr. Yamaguchi.
The flexibility of the new reaction method allows it to work with a wide variety of reactants, including alcohols, phenols, amines, and thiols. This technique is particularly notable because it enables seven different types of chemical transformations turning compounds into thioethers, aryl groups, hydrogen, α-aryls, methylphosphonyl groups, amines, and ethers. This broad range of capabilities makes the method highly adaptable and useful for various chemical processes, improving how aromatic ketones can be used across different industries.
Furthermore, the catalyst system employed in this reaction has shown notable stability and reusability, making the process scalable for industrial applications. “This reaction represents the first successful example of directly catalyzing aromatic ketones without the use of directing groups, setting the stage for future advancements in synthetic chemistry,” explains Dr. Yamaguchi.
This groundbreaking approach represents a significant leap forward in synthetic chemistry, offering a more streamlined, cost-effective, and multifaceted method for utilizing aromatic ketones. As the method continues to be explored and refined, it promises to play a crucial role in advancing the field of synthetic chemistry and beyond.
***
Reference
Authors: Hikaru Nakahara1, Ryota Isshiki1, Masayuki Kubo1, Keiichiro Iizumi1, Kei Muto2, and Junichiro Yamaguchi1
Title of original paper: Versatile deacylative cross-coupling of aromatic ketones
Journal: Chem
DOI: https://doi.org/10.1016/j.chempr.2024.07.002
Affiliations
1Department of Applied Chemistry, Waseda University
2Waseda Institute for Advanced Study, Waseda University
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
About Professor Junichiro Yamaguchi
Junichiro Yamaguchi is a Professor at the Faculty of Science and Engineering at Waseda University. He earned his Ph.D. from Tokyo University of Science in 2007 and gained postdoctoral experience at The Scripps Research Institute. Dr. Yamaguchi began his academic career at Nagoya University as an assistant professor before joining Waseda University in 2016, where he was promoted to full professor in 2018. His research focuses on chemistry with a special interest in making complex topics engaging for students. He also leads the Chem-Station chemistry portal site.
Journal
Chem
DOI
10.1016/j.chempr.2024.07.002
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Versatile deacylative cross-coupling of aromatic ketones
Article Publication Date
12-Sep-2024
COI Statement
The authors declare no competing interests.