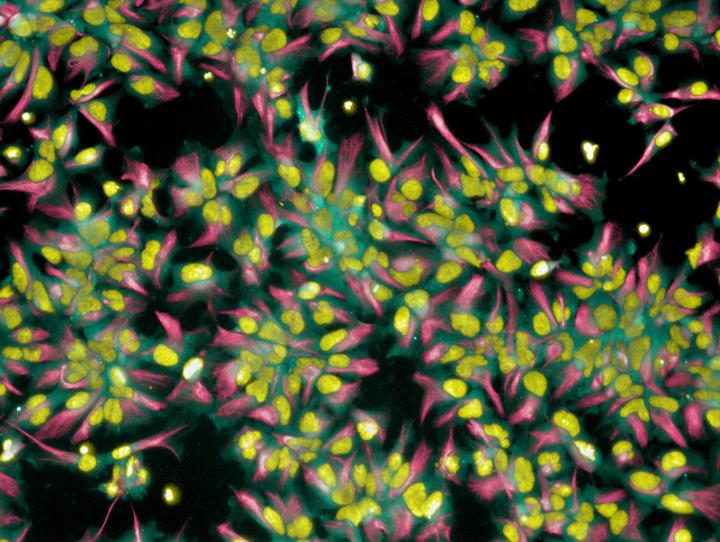
Credit: D'Or Institute for Research and Education (IDOR)
Zika virus (ZIKV) interferes with the cellular machinery controlling cell division and alters the expression of hundreds of genes responsible for guiding the formation and development of brain cells, according to findings released on January 23 by Scientific Reports.
The association between Zika virus (ZIKV) infection and microcephaly has been previously established. Nevertheless, the cellular changes caused by the virus and leading to microcephaly are largely unknown. "Elucidating the foundations of Zika virus infection is crucial in order to develop tools against it", says Stevens Rehen, the principal investigator of the study and a researcher working at the D' Or Institute for Research and Education (IDOR) and at the Institute of Biomedical Sciences at Federal University of Rio de Janeiro (UFRJ) in Brazil.
In a previous study published by the group in Science magazine, researchers observed that the pool of human neural stem cells infected by the Brazilian strain of Zika virus was rapidly and completely depleted, if compared to non-infected cells. This finding led the group to further investigate how Zika virus disrupts the interactome map (or molecular fingerprinting) of infected cells – which is the entire set of cellular and molecular interactions in a given cell group. The analysis of the interactome of Zika-infected cells may reveal the cellular targets and pathways with which the virus interacts or which it modulates, offering valuable opportunities for drug design.
To this end, human neural cells were infected by a strain of ZIKV obtained from a Brazilian patient. These cells were then made into neurospheres, which are organized 3D aggregates of neural cells resembling fetal brain tissue that recapitulate many of the normal early and crucial processes that the brain undergoes through development and thus are a great model for studying the human brain. Next, the group identified the molecular fingerprinting of infected and non-infected cells by checking the expression level and status of innumerous genes and proteins.
The analysis revealed that more than 500 proteins in infected neurospheres had their expression level or status (upregulated vs downregulated) altered, if compared to non-infected neurospheres. A number of these altered proteins are normally involved with tasks such as fixing DNA damage or assuring chromosomal stability. Also, proteins that are normally required for cell growth were silent in infected neurospheres, which may explain why Zika-infected cells die much sooner than their non-infected counterparts. Interestingly, genes driving cell specialization were also silent in infected neurospheres, precluding that specialized brain cells were generated. On the other hand, proteins associated with viral replication were over-abundant, most likely the result of a strategy adopted by the virus to promote its own replication in the host cell. A complete list of all human proteins that have been found altered in Zika-infected neurospheres is available in the study entitled "Zika virus disrupts molecular fingerprinting of human neurospheres", published in Scientific Reports this week.
According to Patricia Garcez, Assistant Professor at the Federal University of Rio de Janeiro and the first author of the study: "these findings provide insights into the molecular mechanisms of ZIKV infection over the course of brain development and may explain some of the consequences seen in the brain of newborns with microcephaly".
###
The study was done at IDOR, in collaboration with researchers from UFRJ, University of Campinas (UNICAMP), Evandro Chagas Institute, Institute Oswaldo Cruz (FIOCRUZ) and Federal University of Para. Funding was provided by the Brazilian Development Bank (BNDES); Funding Authority for Studies and Projects (FINEP); National Council of Scientific and Technological Development (CNPq); Foundation for Research Support in the State of Rio de Janeiro (FAPERJ); Sao Paulo Research Foundation (FAPESP) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Media Contact
Cinthia Fonseca
[email protected]
5521-3478-3110
@institutodor
www.idor.org