Professor Kotohiro Nomura’s research group at Tokyo Metropolitan University developed two high-performance catalysts for efficient synthesis of value-added chemicals (fine chemicals, monomers) from polyester(1) and vegetable oil(2). Their major finding is that simply heating a mixture of polyester and alcohol could afford raw materials exclusively.
Credit: Kotohiro Nomura
Professor Kotohiro Nomura’s research group at Tokyo Metropolitan University developed two high-performance catalysts for efficient synthesis of value-added chemicals (fine chemicals, monomers) from polyester(1) and vegetable oil(2). Their major finding is that simply heating a mixture of polyester and alcohol could afford raw materials exclusively.
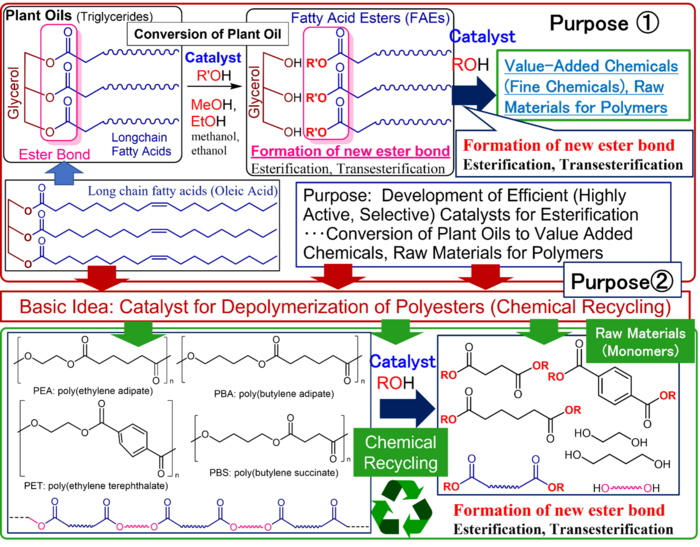
Plastic waste is an enormous environmental problem that needs to be solved immediately, but the amount of plastic being reused (material recycling) is still low, especially chemically recycled into raw materials (called chemical recycling(3)) is currently extremely low. Polyesters, which consist of repeated “ester bonds”(1) formed by the reaction of carboxylic acid and alcohol, is used in plastic bottles and clothing. If these ester bonds could be completely severed, polyester could be converted back into its raw materials. However, conventional methods require high temperatures and large amounts of acidic and/or basic materials. Therefore, a simple, inexpensive, and environmentally friendly method is desired.
The research group developed catalysts to facilitate the synthesis of high value-added chemicals (i.e., fine chemicals) such as the raw materials for polymers, detergents, and cosmetics from inedible vegetable oils, and discovered two types of high-performance catalysts: a calcium oxide catalyst and a titanium catalyst. These catalysts are effective in breaking down polyester based on the same chemical reaction (transesterification) and have been shown to be capable of converting polyester into raw materials with nearly 100% selectivity.(4)
Calcium oxide, which is cheap and easy to acquire, has a proven track record in industrial applications. However, this research group’s idea of applying a catalyst used for converting vegetable oil esters directly as a polyester cracking catalyst is unprecedented in the research literature. Moreover, it’s implementation is inexpensive with a low environmental impact. Furthermore, because these titanium catalysts with their excellent catalyst performance enabled a wide range of applications, strongly suggesting a promise in the synthesis of polymer materials and various fine chemicals from vegetable oil, as well as upcycling(5) from plastic waste to high value-added chemicals.
This research was conducted under the auspices of JST’s Strategic Basic Research Programs and Strategic International Collaborative Research Program (SICORP). The research involving the calcium oxide catalyst was conducted in collaboration with Professor Boonyarach and Professor Kitiyanan of the Petroleum and Petrochemical College, Chulalongkorn University, Thailand. Both serve as Thai co-investigators of an international joint research project in SICORP, which is supported by both JST and the National Science and Technology Development Agency of Thailand (NSTDA).
The joint research paper is as follows: “CaO Catalyzed Transesterification of Ethyl 10-Undecenoate as a Model Reaction for Efficient Conversion of Plant Oils and Their Application to Depolymerization of Aliphatic Polyesters”, ACS Sustainable Chemistry & Engineering. Published online, September 15, 2022, Doi: 10.1021/acssuschemeng.2c04287
(1) Polyester, Ester Bonds
The chemical bond (R’COOR) formed by dehydration of the hydroxyl group (ROH) of an alcohol and the carboxy group (R’COOH) of a carboxylic acid is called an ester bond, and a polymer formed of repeating units of ester bonds is called a polyester.
(2) Vegetable Oil
Vegetable Oils are obtained by extracting and refining the lipids contained in plants. The main component of the fats and oils is triglyceride, which is an alcohol with three carbon atoms called “glycerin” and a carboxylic acid with a chain structure of multiple carbon atoms, collectively called “long-chain fatty acids,” connected by ester bonds. Long-chain (saturated and unsaturated) fatty acids are obtained through the decomposition of glycerides (ester exchange reaction and transesterification with alcohols).
(3) Chemical Recycling
A recycling method in which used resources are chemically treated and converted into other chemical substances for reuse. In this case, it means converting used plastics into raw materials before manufacturing (synthesis).
(4) Selectivity
The ratio of target compound to reacted substrate (raw material).
(5) Up-cycling
Upgrading waste or unused products that would otherwise have been discarded into new products. In this case, it means the conversion of used plastics into chemicals with more added value than raw material.
Journal
ACS Sustainable Chemistry & Engineering
DOI
10.1021/acssuschemeng.2c04877
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Transesterification of Methyl-10-undecenoate and Poly(ethylene adipate) Catalyzed by (Cyclopentadienyl)titanium Trichlorides as Model Chemical Conversions of Plant Oils and Acid-, Base-Free Chemical Recycling of Aliphatic Polyesters
Article Publication Date
15-Sep-2022
COI Statement
None




