This study is led by Prof. Lan Zhu (Department of Obstetrics and Gynecology, Peking Union Medical College Hospital), Prof. Tao Tan (State Key Laboratory of Primate Biomedical Research, Kunming University of Science and Technology), and Prof. Weizhi Ji (State Key Laboratory of Primate Biomedical Research, Kunming University of Science and Technology).
Credit: ©Science China Press
This study is led by Prof. Lan Zhu (Department of Obstetrics and Gynecology, Peking Union Medical College Hospital), Prof. Tao Tan (State Key Laboratory of Primate Biomedical Research, Kunming University of Science and Technology), and Prof. Weizhi Ji (State Key Laboratory of Primate Biomedical Research, Kunming University of Science and Technology).
Animal models are urgently needed to evaluate the host tissue responses and safety of newly developed treatments for pelvic organ prolapse (POP). However, there is a lack of suitable animal models for studying POP due to the unique pelvic anatomy and physiology of humans.
The current POP models include rats, rabbits, sheep, rhesus macaques, and squirrel monkeys. Several methods have been adopted to mimic the pathological state of POP. However, these animals are quadrupeds and have different anatomy, pelvic floor musculature, and birth processes than humans, so the changes induced by these models do not achieve the comparable structural and functional changes in the pelvic floor that are caused by multiple high-risk factors in humans.
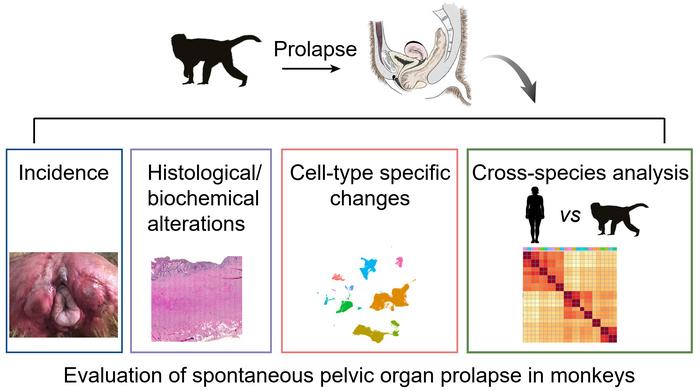
Notably, spontaneous POP has been reported in nonhuman primates; however, the occurrence of spontaneous POP in elderly rhesus macaques has only been sporadically observed without a comprehensive evaluation or in-depth study. Furthermore, the pathological changes and molecular mechanisms of POP in monkeys and the correlations between human and monkey POP are unknown.
This study examined 72 postmenopausal rhesus macaques (aged 12–28 years) from two regions and evaluated the histopathological changes in the prolapsed vaginal wall in nine monkeys. The incidence of spontaneous POP in monkeys is similar to that of women aged 50-59 years. Notably, the histological changes in each layer between the POP and control samples were similar in humans and monkeys. α-SMA expression decreased in POP compared with that in the control group, and the POP smooth muscle cells were disorganized and displaced by increased collagen III deposition. The collagen I/III ratio decreased significantly in the lamina propria layer of the prolapsed vaginal wall of the monkeys. Moreover, elastin fiber content decreased significantly in the prolapsed vaginal wall compared with the control vaginal wall.
Next, single cell RNA sequencing was performed to delineate the molecular regulation of POP in monkeys. The results showed that almost all cell types of the human and monkey overlapped and were strongly correlated. The terms extracellular matrix (ECM) organization and immune reaction were enriched in differentially expressed genes (DEGs) in almost all nonimmune and immune cell types in human and monkey prolapsed vaginas. The results revealed ECM dysregulation and immune disorder may be the conserved mechanisms involved in the pathological process of prolapse in both humans and monkeys.
In conclusion, this study included the largest number of experimental animals to date and uncovered the conserved and species-specific molecular pathways underlying the genesis of POP between humans and monkeys. It demonstrated the feasibility and suitability of spontaneous POP in rhesus macaques as an ideal animal model for POP research.
Journal
Science Bulletin
DOI
10.1016/j.scib.2023.09.003




