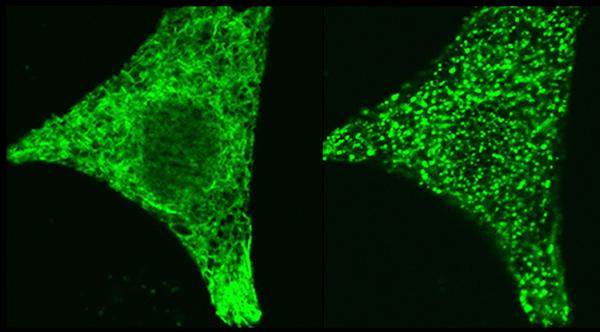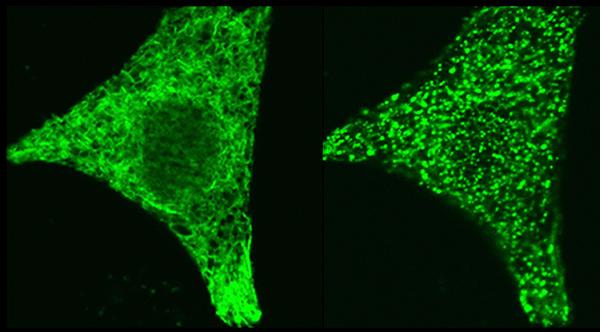
Credit: Dr. Aparna Gudlur, La Jolla Institute for Immunology
LA JOLLA, CA–All mammalian cells need a ready supply of calcium ions to execute functions as diverse as neurotransmission, muscle contraction, hormone release, or immune responses. So fundamental is this requirement that cells protect themselves from disaster by storing calcium in a network of intracellular cisterns called the endoplasmic reticulum, or ER. Then, as cells lose calcium as part of their daily routine, channels open in the cell's membrane allowing calcium influx from outside to re-fill the ER reserves and maintain calcium-driven cellular functions.
Two studies recently published by La Jolla Institute for Immunology (LJI) investigators Patrick Hogan, PhD, and Aparna Gudlur, PhD–one paper appearing early this year in Cell Reports and the other in the October 31, 2018, issue of Nature Communications–report how a calcium-sensing protein called STIM1 signals that it's time to initiate calcium retrieval and then relays that message to its partner, the calcium channel ORAI. This body of work lays the groundwork for novel ways to manipulate aberrant calcium signaling in the immune system, particularly in the context of autoimmune or inflammatory disease.
"We've known for a decade that STIM1 protein moved toward the plasma membrane to open ORAI channels when ER calcium levels drop," says Hogan, a professor in the Division of Signaling and Gene Expression. "Our recent work shows how the STIM machinery functions on a molecular level. Understanding these mechanisms is critical, as calcium is important for a panoply of immune responses."
The Cell Reports paper reveals how STIM1 protein elongates as it switches from a resting to an activated state when calcium reserves drop. STIM1 is a transmembrane protein that spans the ER wall: one end pokes a calcium-sensing dipstick-like tail inside the reserve; in a resting, calcium-replete state, the other arm juts out from the ER but remains tucked up against the ER wall, out of touch with dormant ORA1 channels that dot the cell membrane.
The group defined what happens when calcium levels fall by constructing and testing the behavior of genetically-modified STIM1 proteins in cultured cells. Analysis showed that calcium loss from the sensor tail caused the membrane-spanning regions of pairs of STIM1 proteins to pull together inside the ER wall, prompting the outer arms to extend toward the cell membrane. That shape change brought STIM1 close enough to ORAI channels to reach out and open them, allowing calcium to flow back into cells.
"The first paper showed how STIM1 communicates with a channel protein in the plasma membrane via a structural change," says Hogan. "Our latest paper takes that process a step back in time and reveals how calcium loss inside the ER initiates that change."
Put simply, the more recent paper focuses on the workings of STIM1's calcium-sensing dipstick. Previously, protein chemists found that calcium inside the ER binds to a structural motif in the STIM1 tail called an "EF-hand" and proposed that when the hand was empty, STIM1 activated ORAI. Gudlur, an Instructor in the Hogan lab and the new paper's first author, explains that it isn't quite that simple. "We now report that multiple calciums bind not only to the EF-hand but to other sites in the STIM1 ER domain, and that the sites depend on each other," she says. "That revises the concept of how STIM1 gets activated."
Not only that, but according to Hogan the prevailing belief had been that calcium dissociation causes STIM1's dipstick-like region inside the ER to unfold dramatically and assume a chaotic structure, a disorderliness deemed necessary for ORAI activation. "We now show that when calcium is low in the ER this region doesn't unfold but instead acquires a novel but stable structure that favors interaction with the ORAI channel," he says. "That's important because it means that interaction could be targeted by inhibitors."
In fact, drug companies have already sought to develop drugs to block ORAI calcium channels as means to halt calcium-dependent immune responses in autoimmune disease or in inflammatory conditions like acute pancreatitis. Some ORAI-blocking drugs are currently in Phase I and II clinical trials and thus far appear non-toxic. But their therapeutic potential remains unknown.
Gudlur, who had a leading role in both studies, speculates that developing direct ORAI/STIM1 blockers as therapies may be just an initial step, as both proteins are common to many cell types. "Targeting ORAI channels as a way to block excess calcium signaling only in certain cells may require greater specificity," she says. "Our work is a first step in identifying cell-specific factors capable of modulating ORAI/STIM1 activity. Once we find them, drug screens targeting those factors would be called for."
Hogan agrees that drug discovery is built on a foundation of exploration. "People conducting basic research must examine fundamental cellular processes," he says, noting that more than 20 years passed between the discovery of the immune checkpoint protein CTLA4 and FDA approval of immune checkpoint blockade as treatment for melanoma. (Note: four days after this interview the "discoverers" named by Hogan were awarded the 2018 Nobel Prize in Physiology and Medicine). "If you don't tinker with the basics of a cell you will never jump forward. We don't always know where therapies are going to come from."
###
The study was funded by the La Jolla Institute, by NIH grants AI084167, GM110397, AI040127, S10OD21724, and GM112003, GM126532, postdoctoral fellowship 2016/12505-8 from the São Paulo Research Foundation (FAPESP), an American Cancer Society grant RSG-16-215-01 TBE and by National Natural Science Foundation of China grant NSFC-31471279 and Fundamental Research Funds for the Central Universities 2017EYT21.
Full citation:
Nupura Hirve, Vangipurapu Rajanikanth, Patrick G. Hogan, Aparna Gudlur. "Coiled-Coil Formation Conveys a STIM1 Signal from ER Lumen to Cytoplasm", Cell Reports, 2018 DOI:10.1016/j.celrep.2017.12.030
Aparna Gudlur, Ana Eliza Zeraik, Nupura Hirve, V Rajanikanth, Andrey A Bobkov, Guolin Ma, Sisi Zheng, Youjun Wang, Yubin Zhou, Elizabeth A Komives, Patrick G Hogan. "Calcium sensing by the STIM1 ER-luminal domain", Nature Communications, 2018. DOI: 10.1038/s41467-018-06816-8
About La Jolla Institute for Immunology
The La Jolla Institute for Immunology is dedicated to understanding the intricacies and power of the immune system so that we may apply that knowledge to promote human health and prevent a wide range of diseases. Since its founding in 1988 as an independent, nonprofit research organization, the Institute has made numerous advances leading toward its goal: life without disease.
Media Contact
[email protected]
[email protected]
858-357-7481
@ljiresearch
www.lji.org
Related Journal Article
http://dx.doi.org/10.1038/s41467-018-06816-8