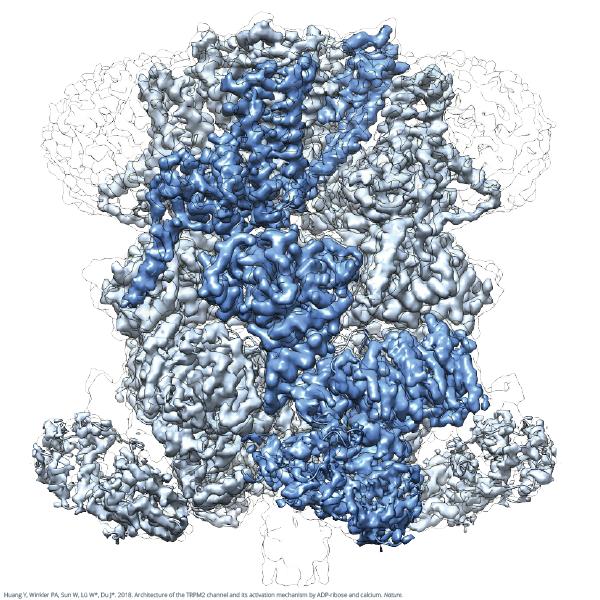
Credit: Wei Lü and Juan Du
GRAND RAPIDS, Mich. (September 24, 2018) — A team led by Van Andel Research Institute (VARI) scientists has revealed for the first time the atomic-level structure of TRPM2, a protein that may be a promising drug target for conditions such as Alzheimer's disease and bipolar disorder.
TRPM2 is found throughout the body and is integrally involved in regulating core body temperature, mediating immune responses and governing apoptosis, the programmed death of cells. It is activated by range of stimuli including oxidative stress, which results from chemical imbalances and is linked to numerous neurological conditions.
"TRPM2 is absolutely critical for healthy function but, until today, we were missing key insights into its structure and mechanism of action," said Juan Du, Ph.D., an assistant professor at VARI and co-senior author on a study describing TRPM2's structure, published today in Nature "It is our hope that these findings will act as a schematic for designing better and desperately needed medications for a host of neurological conditions."
The atomic-level images of TRPM2 depict a bell-like structure, with a transmembrane domain region on the bell's shoulder and an expanded NUDT9-H domain on the bell's lip. They also reveal a novel drug binding site for ADPR, a messenger molecule associated with oxidative stress and metabolism, which overturns the prevailing view that TRPM2 binds ADPR at the NUDT9-H domain. These revelations provide valuable details that could inform the design of therapeutic drugs to treat temperature-related diseases and prevent neuronal death in neurodegenerative diseases.
"Although we have known for some time that TRPM2 is a vital component of many biological processes and a possible drug target, we didn't know exactly what it looked like or how it worked," said Wei Lü, Ph.D., an assistant professor at VARI and co-senior author. "Today's findings change that, and go a long way toward a more comprehensive understanding of these incredibly important molecules."
TRPM2 belongs to the TRP superfamily, a group of proteins that mediate responses to sensory stimuli, such as pain, pressure, vision, temperature and taste. Broadly known as ion channels, proteins like TRP nestle within cells' membranes, acting as gatekeepers for chemical signals passing into and out of the cell. The eight proteins that comprise the TRPM subfamily are part of this broader group.
To date, TRPM2 is the fourth TRPM protein to have its structure resolved at the atomic level and the second TRPM protein to be determined at atomic resolution at VARI. In December, Lü and Du revealed the structure of TRPM4, which plays a role in regulating blood supply to the brain. It was the first structure of a TRPM protein determined at atomic resolution.
The findings were made possible by VARI's state-of-the-art David Van Andel Advanced Cryo-Electron Microscopy Suite, which allows scientists to view some of life's smallest components in exquisite detail. VARI's largest microscope, the Titan Krios, is so powerful that it can visualize molecules 1/10,000th the width of a human hair.
###
In addition to Du and Lü, authors include Yihe Huang, Ph.D., and Paige A. Winkler, Ph.D., both postdoctoral fellows in Lü and Du's labs, and Weinan Sun, Ph.D., a postdoctoral associate in the Spruston Lab at Howard Hughes Medical Institute's Janelia Research Campus.
Media Contact
Beth Hinshaw Hall
[email protected]
616-234-5519
Related Journal Article
http://dx.doi.org/10.1038/s41586-018-0558-4





