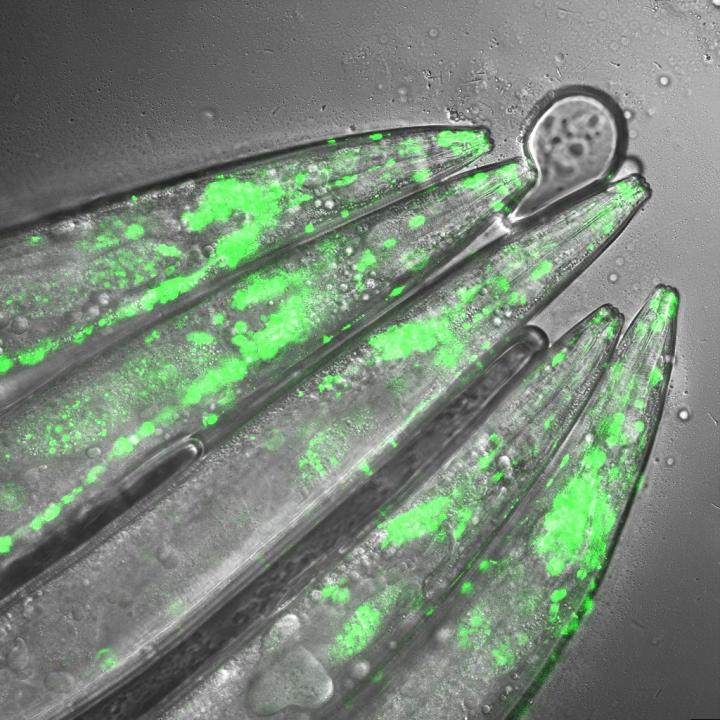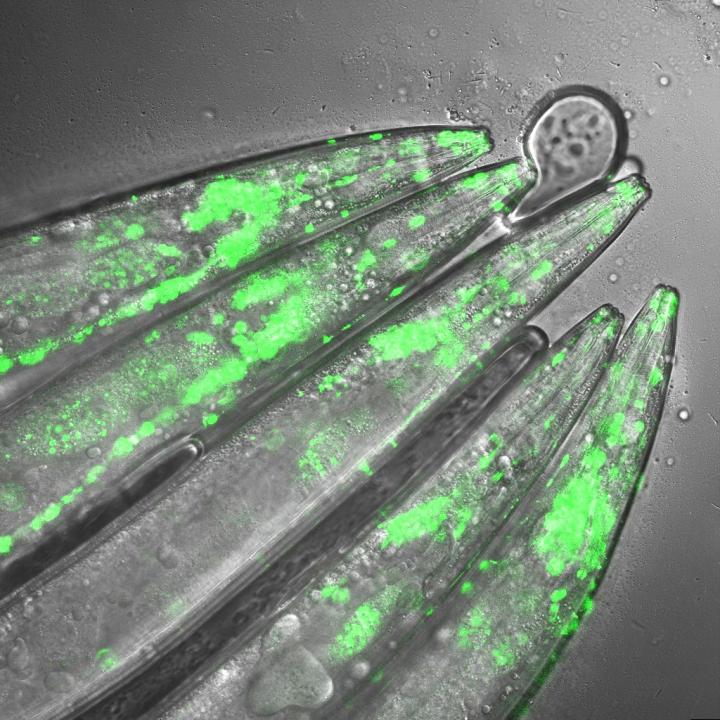
Credit: Seda Koyuncu and Isabel Saez
The neuroscientist Dr David Vilchez and his team at CECAD, the University of Cologne's Cluster of Excellence for Aging Research, have made an important step towards understanding the mechanisms that cause the neurodegenerative disorder Huntington's disease. Particularly, they identified a system blocking the accumulation of toxin protein aggregates, which are responsible for neurodegeneration. The results have now been published in the journal 'Nature Communications'.
Huntington's disease is a neurodegenerative disorder that results in the death of brain cells, leading to uncontrolled body movement, loss of speech and psychosis. Mutations in the huntingtin gene cause the disease, resulting in the toxic aggregation of the huntingtin protein. The accumulation of these aggregates causes neurodegeneration and usually leads to the patient's death within twenty years after the onset of the disease.
To examine the mechanisms underlying Huntington's disease, Vilchez and his team used so-called induced pluripotent stem cells (iPSC) from Huntington's disease patients, which are able to differentiate into any cell type, such as neurons. Induced pluripotent stem cells derived from patients with Huntington's disease exhibit a striking ability to avoid the accumulation of toxic protein aggregates, a hallmark of the disease. Even though iPSCs express the mutant gene responsible for Huntington's disease, no aggregates were found.
The researchers identified a protein called UBR5 as a protective mechanism for the cells, promoting the degradation of mutant huntingtin. These findings can contribute to a better understanding of Huntington's disease and could be a step stone to developing further treatment in patients.
The researchers screened immortal iPSCs from patients and derived neurons for differences in their ability to avoid mutant huntingtin aggregation. They found that huntingtin can be degraded by the cellular disposal system known as the proteasome. However, this system is defective in the neurons, which leads to the aberrant aggregation of the mutant huntingtin protein. Vilchez and his team found that UBR5 is increased in pluripotent stem cells to accelerate the degradation of huntingtin in the cells. To examine the role of UBR5 in the regulation of the mutant huntingtin gene (HTT), they reduced the levels of UBR5 and could immediately see an accumulation of aggregated proteins in iPSCs. 'This was striking to see', says Vilchez. 'From nothing, the cells went to huge amounts of aggregates.'
The authors went a step further and examined whether UBR5 also controls mutant huntingtin aggregation in Huntington's disease organismal models. Indeed, they found that dysregulation of UBR5 results in a massive increase in the aggregation and neurotoxic effects in neurons. On the other hand, promoting UBR5 activity blocks mutant huntingtin aggregation in the Huntington's disease models.
To test the specificity of the results, the researchers also kept an eye on other illnesses. 'We also checked the mechanism in other neurodegenerative diseases like amyotrophic lateral sclerosis', says Seda Koyuncu, a doctoral student working in Vilchez's lab and a main author of the publication. 'Our result is very specific to Huntington's disease', adds Dr Isabel Saez, another main author working with Vilchez at CECAD. Even though the results could be important for treatment and drug development, there is no therapy yet. 'It's not like you discover something new and then there is a cure, it's more difficult – but in some years there might be a therapy', Saez comments. Until then, more research needs to be done.
###
CECAD is the Cluster of Excellence at the University of Cologne and Cologne University Hospital conducting research on diseases associated with aging. Its six research areas cover a wide range of basic research on the causes and mechanisms of these diseases. David Vilchez is a Principal Investigator in Research Area B: 'Disruptions in Protein Metabolism Cause Aging-Associated Diseases'. His research mainly focuses on protein homeostasis regulation in stem cells and aging.
Publication:
'The ubiquitin ligase UBR5 suppresses proteostasis collapse in pluripotent stem cells from Huntington's disease patients'. Seda Koyuncu, Isabel Saez, Hyun Ju Lee, Ricardo Gutierrez-Garcia, Wojciech Pokrzywa, Azra Fatima, Thorsten Hoppe and David Vilchez. Nature Communications, volume 9, 23 July 2018, article number: 2886 (2018)
https://www.nature.com/articles/s41467-018-05320-3
Media Enquiries:
Dr David Vilchez
Principal Investigator/Junior Research Group Leader, CECAD Cologne
+49 221 478 84172
[email protected]
Press and Communications Team:
Peter Kohl
+49 221 478-84043
[email protected]
More information:
http://www.cecad.uni-koeln.de/home
Media Contact
David Vilchez
[email protected]
49-221-478-84172
@UniCologne
http://www.uni-koeln.de
Related Journal Article
http://dx.doi.org/10.1038/s41467-018-05320-3