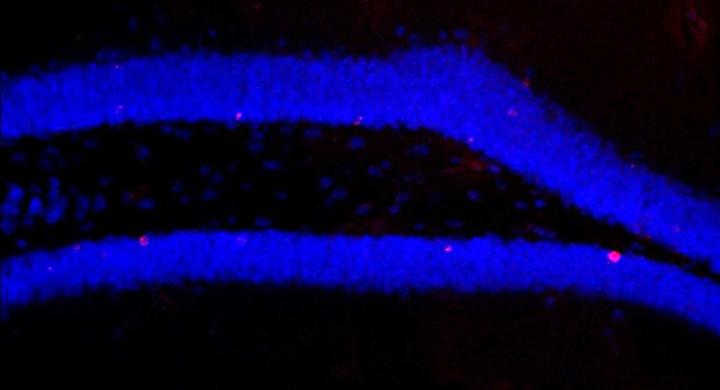
A special kind of immune cell serves as an intermediary between gut bacteria and the brain. Dr. Susanne Wolf of the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) discovered this in tests on mice and published her findings in the journal Cell Reports. The research findings are of significance when it comes to the effects of using antibiotics in the long term, and could also help to alleviate the symptoms of mental disorders.
The gut and the brain “talk” to one another via hormones, metabolic products or direct neural connections. A specific population of monocyte immune cells acts as a further link between the two, as Dr. Susanne Wolf from the MDC research group led by Prof. Helmut Kettenmann recently discovered in collaboration with colleagues from the University of Magdeburg, the Charité – Universitätsmedizin Berlin, and the US National Institutes of Health (NIH).
The researchers switched off the gut microbiome in mice, i.e. their intestinal bacteria, with a strong concoction of antibiotics. Compared to the mice that had not undergone treatment, they subsequently observed significantly fewer newly formed nerve cells in the hippocampus region of the brain. The memory of the treated mice also deteriorated because the formation of these new brain cells – a process known as neurogenesis – is important for certain memory functions.
As well as impaired neurogenesis, the researchers also found that the population of a specific immune cell in the brain – the Ly6C(hi) monocytes – decreased significantly when the microbiota was switched off. Could these immune cells be a previously unknown intermediary between the two organ systems? Wolf and her team tested and confirmed this hypothesis: when they removed just these cells from the mice, neurogenesis declined and when they gave the cells to the mice that had been on antibiotics, neurogenesis increased once again.
The researchers cured the antibiotic-treated mice using two different strategies: the mice were either given a mixture of selected bacterial strains or had access to voluntary training in the running wheel, thus reversing the negative effects of the antibiotics. The mice’s number of monocytes increased and their memory performance and neurogenesis improved. However, it was not possible to restore the immune and brain functions using the microbiota of untreated mice.
According to Wolf, the previously unknown intermediary function of the immune cells is of particular scientific interest: “With the Ly6C(hi) monocytes, we may have discovered a new general communication path from the periphery to the brain.”
Applied to humans, the findings do not show that all antibiotics disrupt brain function, as the combination of drugs used in the study was extremely potent. “It is possible, however, that similar effects could result from treatments involving long-term use of antibiotics,” says Wolf. The research team also found that the antibiotics may affect neurogenesis directly, and not act only via the gut bacteria.
According to Wolf, the new study is also of significance for treating people with mental disorders such as schizophrenia or depression, who also have impaired neurogenesis: “In addition to medication and physical exercise, these patients could potentially also benefit from probiotic preparations. In order to test this, we would like to conduct clinical pilot studies together with the Charité.”
###
Media Contact
Vera Glaßer
[email protected]
49-309-406-2120
http://www.mdc-berlin.de
The post Healthy intestinal flora keeps the mind sharp — with some help from the immune system appeared first on Scienmag.