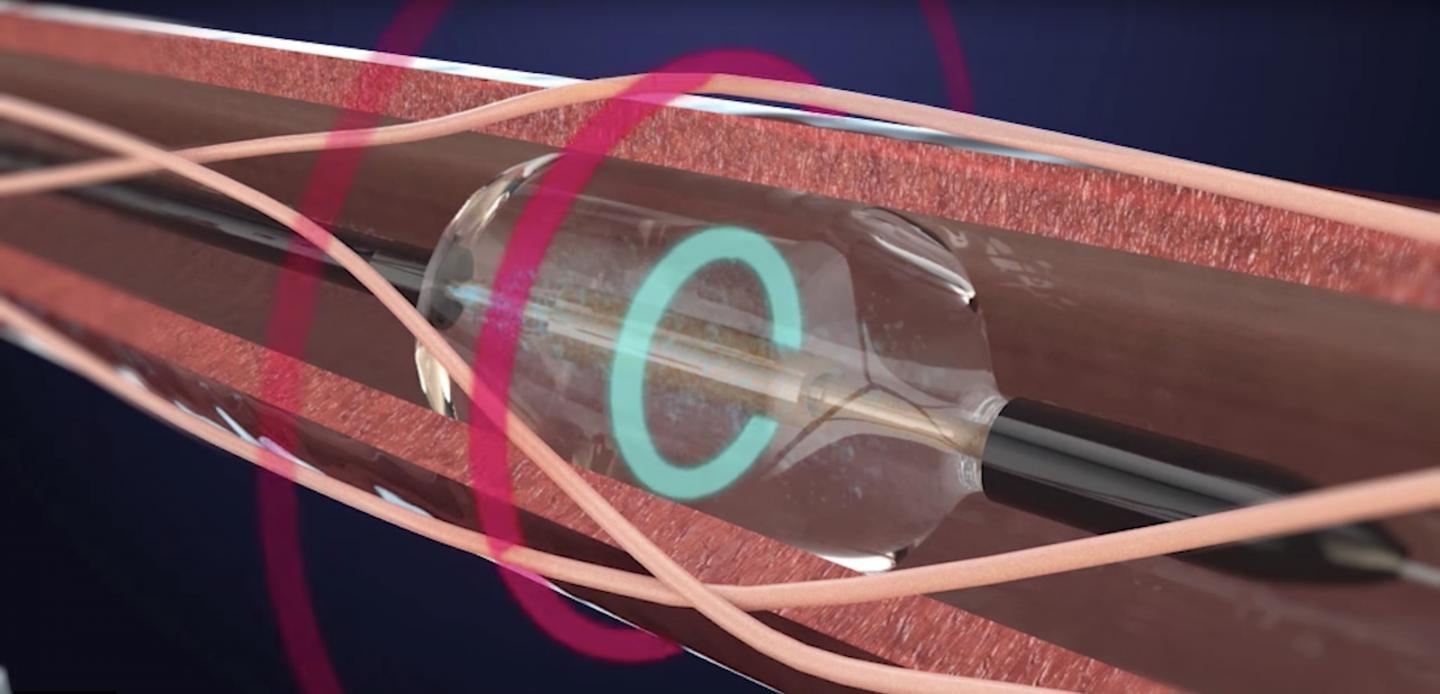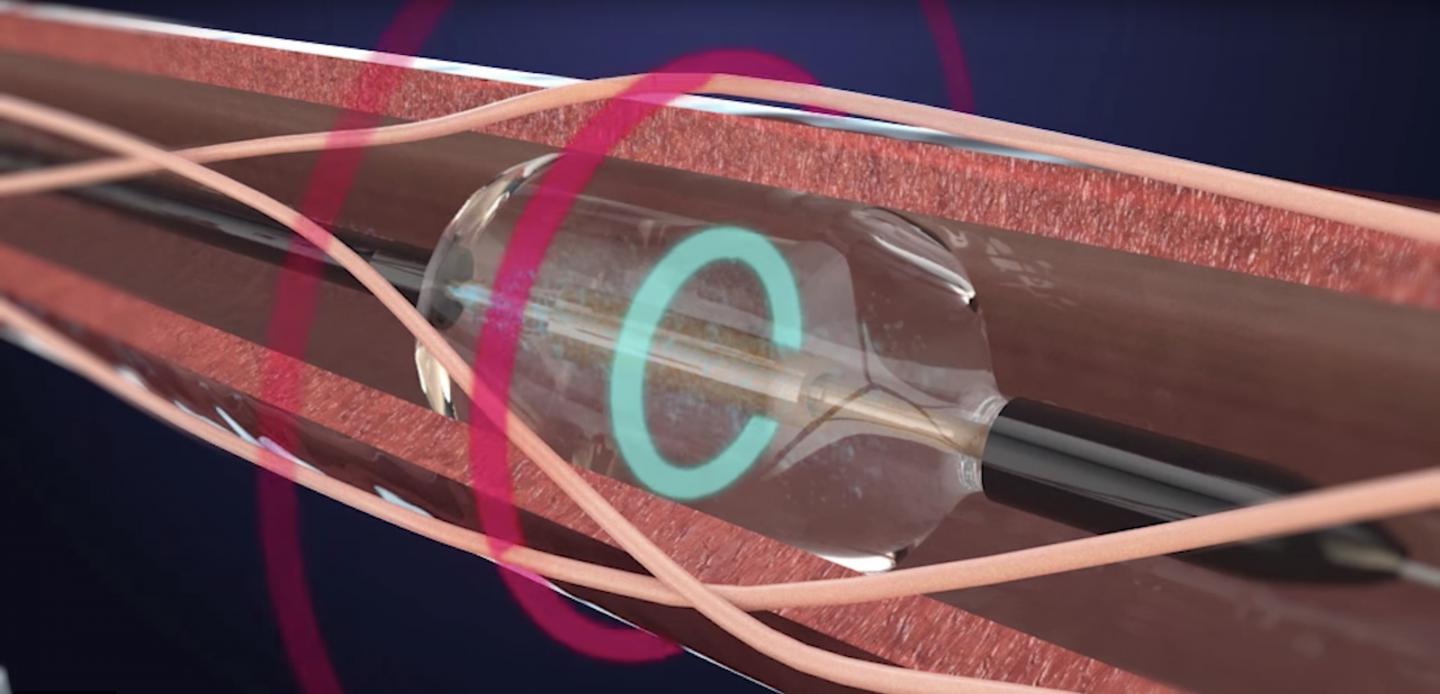
Credit: ReCor Medical, Inc.
An operation that targets the nerves connected to the kidney has been found to significantly reduce blood pressure in patients with hypertension, according to the results of a clinical trial led in the UK by Queen Mary University of London and Barts Health NHS Trust, and supported by the National Institute for Health Research (NIHR).
The results are published in The Lancet and have been presented at the EuroPCR congress in Paris.
If the findings are confirmed in more extensive clinical trials, the surgery could offer hope to patients with high blood pressure who do not respond to drugs, and are at increased risk of cardiovascular diseases, including stroke and heart attack.
The international clinical trial, carried out from 2017 to 2018 at St Bartholomew's Hospital in the UK by the NIHR Barts Biomedical Research Centre, tested a one-hour operation called 'renal denervation', which uses ultrasound energy to disrupt the nerves between the kidneys and the brain that carry signals for controlling blood pressure.
146 patients in the United States, France, Germany, the Netherlands, Belgium and the United Kingdom were randomised to receive either renal denervation or a 'sham procedure' – the surgical equivalent of a placebo. Patients also remained off blood pressure medications for two months unless specified blood pressure levels were exceeded.
After two months, the renal denervation group experienced an 8.5 mm Hg reduction in blood pressure, which was a 6.3 mm Hg greater reduction compared with the sham group.
More than 66 per cent of subjects treated with renal denervation demonstrated a 5 mm Hg or greater reduction in blood pressure, compared with 33 per cent in the sham group.
No major adverse events were reported in either group, and the blood pressure lowering effect of renal denervation was consistent across sex and ethnicity.
UK Principal Investigator Dr Melvin Lobo from Queen Mary University of London and Barts Health NHS Trust said: "These results leave us clinicians in no doubt that this ultrasound-based therapy works to improve blood pressure control – at least in the short term. Further larger trials will be needed to confirm the efficacy and safety of the technology, but we hope that they could lead to renal denervation therapy being offered as an alternative to lifelong medications for hypertension."
The study has limitations including the short follow-up time of two months. This was done for safety reasons to minimise the duration of patients being off antihypertensive medications. Longer follow-up of this trial and additional numbers of treated patients will be necessary to provide greater assurance of safety and to exclude rare adverse events.
###
The study was funded by ReCor Medical, Inc. which manufactures the Paradise® Renal Denervation System used in the study.
For more information, please contact:
Joel Winston
Public Relations Manager (School of Medicine and Dentistry)
Queen Mary University of London
[email protected]
Tel: +44 (0)20 7882 7943 / +44 (0)7970 096 188
Notes to the editor
- Research paper: 'Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE-HTN SOLO Randomised Trial'. Prof. Michel Azizi*, MD, PhD; Prof. Roland E. Schmieder, MD; Prof. Felix Mahfoud, MD; Prof. Michael A. Weber, MD; Joost Daemen, MD, PhD; Justin Davies, MBBS, MRCP, PhD; Jan Basile, MD; Ajay J. Kirtane, MD, SM; Yale Wang, MD; Melvin D. Lobo, PhD, FRCP; Manish Saxena, MBBS, MSc; Lida Feyz, MD; Florian Rader, MD, MSc; Philipp Lurz, MD; Jeremy Sayer, MD, FRCP; Prof. Marc Sapoval, MD; Terry Levy, MB ChB, FRCP; Kintur Sanghvi, MD; Josephine Abraham, MD, MPH; Andrew SP Sharp, MD; Naomi DL Fisher, MD; Michael J. Bloch, MD; Helen Reeve-Stoffer, PhD; Leslie Coleman, DVM, MS, DACLAM; Christopher Mullin, MS; and Prof. Laura Mauri, MD, MSc on behalf of the RADIANCE-HTN Investigators. The Lancet.
About Barts Health NHS Trust
With a turnover of £1.4 billion and a workforce of around 16,000, Barts Health is the largest NHS trust in the country, and one of Britain's leading healthcare providers. The Trust's five hospitals – St Bartholomew's Hospital in the City, including the Barts Heart Centre, The Royal London Hospital in Whitechapel, Newham University Hospital in Plaistow, Whipps Cross University Hospital in Leytonstone and Mile End – deliver high quality compassionate care to the 2.5 million people of East London and beyond.
About Queen Mary University of London
Queen Mary University of London is one of the UK's leading universities with 25,332 students representing more than 160 nationalities.
A member of the Russell Group, we work across the humanities and social sciences, medicine and dentistry, and science and engineering, with inspirational teaching directly informed by our research. In the most recent national assessment of the quality of research, we were placed ninth in the UK amongst multi-faculty universities (Research Excellence Framework 2014).
As well as our main site at Mile End – which is home to one of the largest self-contained residential campuses in London – we have campuses at Whitechapel, Charterhouse Square, and West Smithfield dedicated to the study of medicine and dentistry, and a base for legal studies at Lincoln's Inn Fields.
Queen Mary began life as the People's Palace, a Victorian philanthropic project designed to bring culture, recreation and education to the people of the East End. We also have roots in Westfield College, one of the first colleges to provide higher education to women; St Bartholomew's Hospital, one of the first public hospitals in Europe; and The London, one of England's first medical schools.
About the National Institute for Health Research
The National Institute for Health Research (NIHR): improving the health and wealth of the nation through research.
Established by the Department of Health and Social Care, the NIHR:
- funds high quality research to improve health
- trains and supports health researchers
- provides world-class research facilities
- works with the life sciences industry and charities to benefit all
- involves patients and the public at every step
About ReCor Medical, Inc.
ReCor Medical is a private, development-stage, medical device company with the Paradise? System, a proprietary, ultrasound-based system for endovascular denervation of the renal nerves (RDN). RDN is a potential therapeutic option for treatment of hypertension, one of the most prevalent medical conditions. The Paradise System bears a CE mark but is not approved for sale in the United States. ReCor is conducting the RADIANCE-HTN clinical trial under an IDE from the US FDA in the United States and Europe. The RADIANCE-HTN SOLO trial is a randomized, sham-controlled, and blinded study of 146 hypertensive patients temporarily withdrawn from their hypertension medications, comparing the ReCor Paradise ultrasound-based Renal Denervation System to a sham procedure.
Media Contact
Joel Winston
[email protected]
44-020-788-27943
@QMUL
http://www.qmul.ac.uk
Related Journal Article
http://dx.doi.org/10.1016/S0140-6736(18)31082-1