Credit: Penn Medicine
PHILADELPHIA – Penn Medicine researchers have used CRISPR/Cas9 gene editing technology to isolate a key genetic feature that could cause resistance to PARP inhibitors in patients with ovarian cancer – and they've also proven they have a way to see that feature using PET imaging.
The team found PARP inhibitors – a type of targeted therapy that kills cancer cells with mutations in their DNA repair genes while sparing healthy tissue that does not have the mutations – specifically require the presence of PARP-1 in order to take effect. They also show that a radioactive tracer developed at Penn makes PARP-1 visible during PET scans and may provide a method of measuring PARP-1 in ovarian cancer that complements biopsy. The findings of this multidisciplinary team – which included radiologists, pathologists, and oncologists from the University of Pennsylvania's Perelman School of Medicine – were published in the Journal of Clinical Investigation this month.
Epithelial ovarian cancer is the fifth deadliest type of cancer in women, with more than 70 percent of patients already showing advanced disease when they're diagnosed. Standard treatment relies on chemotherapy, but more recently, PARP inhibitors have emerged as a way to improve patient outcomes. Three of these drugs are currently approved for use by the U.S. Food and Drug Administration, and patients commonly receive these inhibitors if they test positive for the genetic mutations BRCA1 and BRCA2. However, only about 50 percent of these patients respond.
Penn researchers used CRISPR/Cas9 gene editing in the lab to delete PARP-1 from ovarian cancer cells, then exposed those cells to PARP inhibitors and compared them to a control group with PARP-1. Cells without PARP-1 showed less DNA damage and less responsiveness to the inhibitors than the unedited control group cells. In one case, the loss of PARP-1 resulted in a greater than a 1,000-fold decrease in sensitivity to the PARP inhibitor.
"These findings show that PARP-1 is required for PARP inhibitors to do their job," said the study's co-senior author Mehran Makvandi, PharmD, RPh, BCNP, an instructor of Radiology. "These data also demonstrated the clinical need to evaluate PARP-1 levels in BRCA cancers to better understand which patients are likely to benefit and which may be resistant."
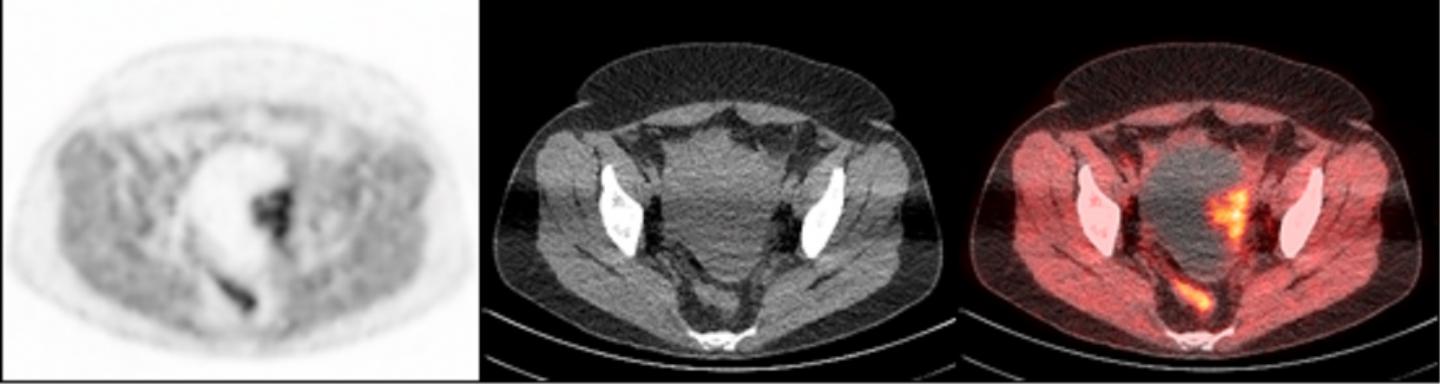
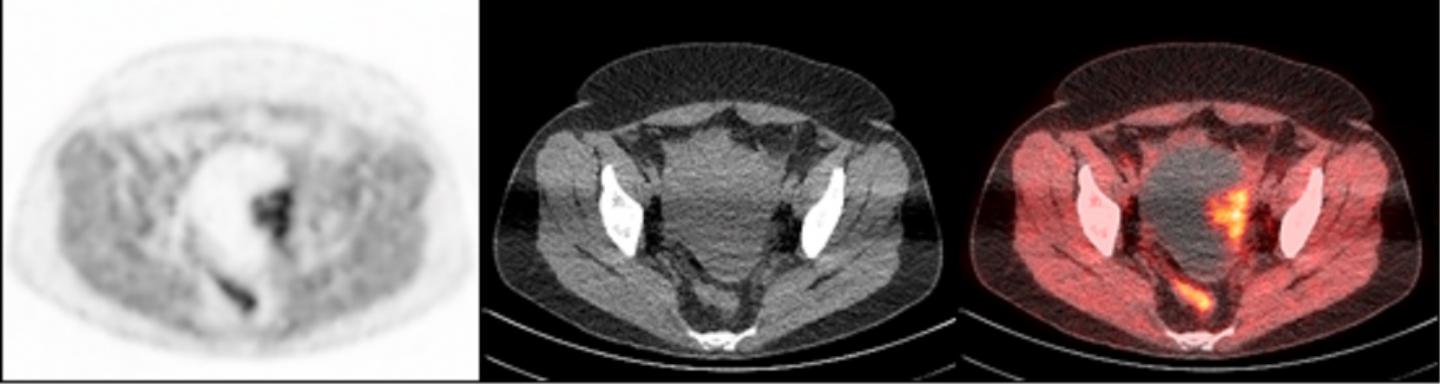
To accomplish this, researchers used a radioactive tracer that binds to PARP-1, making it visible during PET scans. The tracer, called FluorThanatrace, was specially designed by Robert H. Mach, PhD, the Britton Chance Professor of Radiology. After a pre-clinical test using mouse models showed the tracer was hitting its specific molecular target, the study's authors moved to a phase I trial.
A clinical trial involving 10 patients led to 13 tissue samples. These samples had a wide spectrum of uptake of the tracer, but that uptake correlated with PARP-1 expression. In other words, the more the tracer was able to bind to the tumor, the higher the level of PARP-1 that tumor expressed.
"This is proof-of-concept that we not only have a potential biomarker for the effectiveness of PARP inhibitors, but we also have a non-invasive imaging technique to find that biomarker in ovarian cancer patients," Makvandi said.
Researchers say the next step in this work is to evaluate whether this technique can be used to predict efficacy and resistance to PARP inhibitors. Makvandi and Fiona Simpkins, MD, an assistant professor of Obstetrics and Gynecology and co-author on this study, are leading future clinical trials to directly test the predictive value of this approach in ovarian cancer patients receiving PARP inhibitor monotherapy. Trials are also planned in breast cancer.
###
The study's co-senior author was Lilie L. Lin, MD, an adjunct associate professor of Radiation Oncology and an associate professor of Radiation Oncology at University of Texas MD Anderson Cancer Center. Co-authors include David A Mankoff, MD, PhD, the Gerd Muehllehner Professor of Radiology and co-director of the Cancer Imaging Core of Penn's Abramson Cancer Center, Roger A. Greenberg, MD, PhD, a professor of Cancer Biology and director of Basic Science in the Basser Center for BRCA at the Abramson Cancer Center, and Austin Pantel, MD, an Instructor in Radiology.
The study was supported by the Department of Defense (OC160269), a Basser Center team science grant, the National Cancer Institute (R01CA174904), a Department of Energy training grant (DE-SC0012476), Abramson Cancer Center Radiation Oncology pilot grants, the Marsha Rivkin Foundation, the Kaleidoscope of Hope Foundation, and the Paul Calabresi K12 Career Development Award (5K12CA076931).
Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $7.8 billion enterprise.
The Perelman School of Medicine has been ranked among the top medical schools in the United States for more than 20 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $405 million awarded in the 2017 fiscal year.
The University of Pennsylvania Health System's patient care facilities include: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center — which are recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report — Chester County Hospital; Lancaster General Health; Penn Medicine Princeton Health; Penn Wissahickon Hospice; and Pennsylvania Hospital — the nation's first hospital, founded in 1751. Additional affiliated inpatient care facilities and services throughout the Philadelphia region include Good Shepherd Penn Partners, a partnership between Good Shepherd Rehabilitation Network and Penn Medicine, and Princeton House Behavioral Health, a leading provider of highly skilled and compassionate behavioral healthcare.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2017, Penn Medicine provided more than $500 million to benefit our community.
Media Contact
John Infanti
[email protected]
215-301-5221
@PennMedNews
http://www.uphs.upenn.edu/news/
Related Journal Article
http://dx.doi.org/10.1172/JCI97992