Credit: Bruce Crippen for Standard Bariatrics
(Cincinnati, OH– April 18, 2018) Standard Bariatrics, Inc., an innovator of bariatric surgical devices, has raised a Series A round of financing. RiverVest Venture Partners led the round with participation from Hatteras Venture Partners, Queen City Angels/QCA First Fund V, Emergent Medical Partners, Accelerant Fund and North Coast Angels. The size of the round is undisclosed.
"This new capital will enable us to complete our product development plans to launch new products for use in gastric procedures," said Matt Sokany, CEO of Standard Bariatrics.
"The company's objective is to develop tools to assist in making certain surgical procedures more consistent with potentially better clinical outcomes while reducing surgical supply cost."
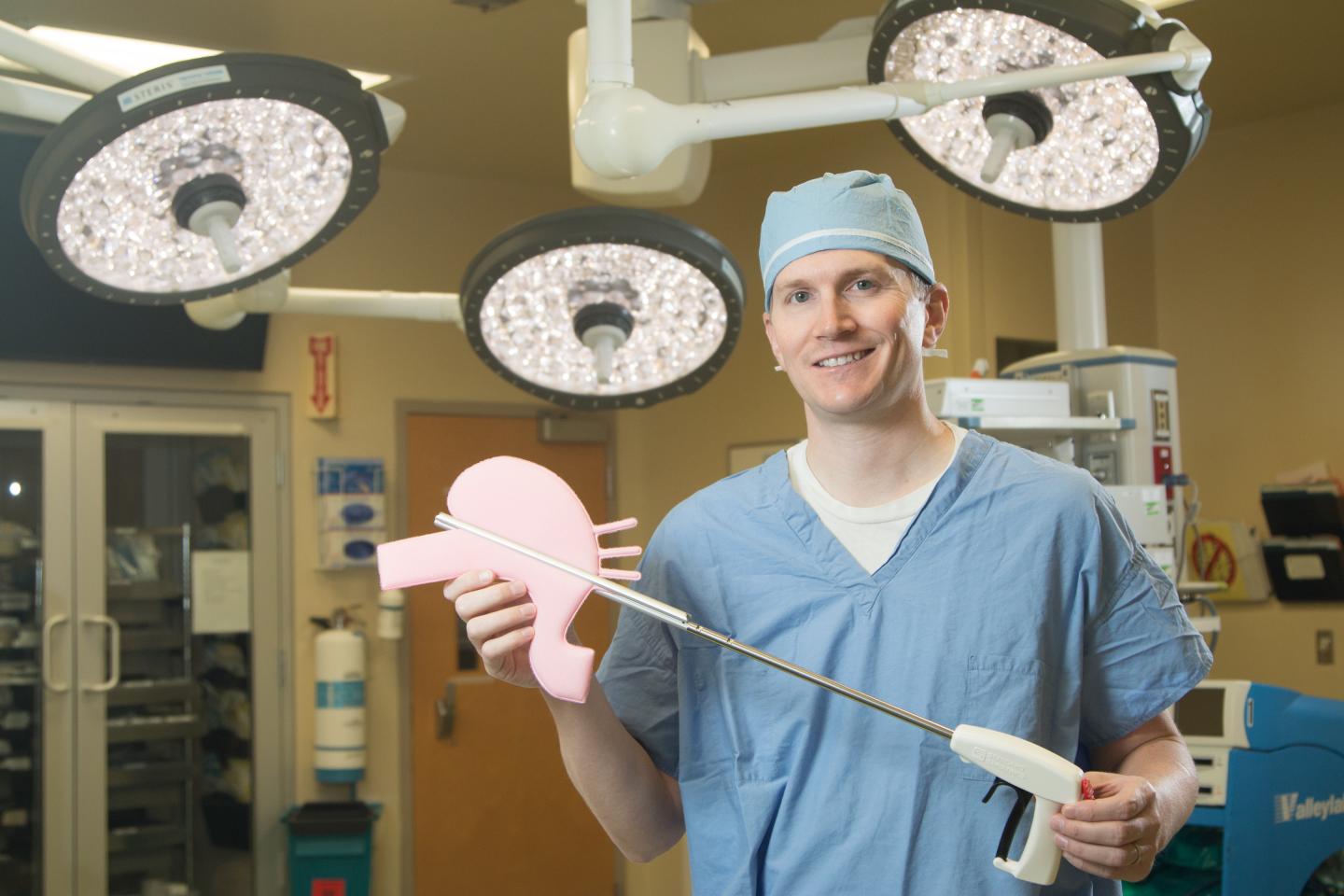
Standard Bariatrics' first device, the STANDARD CLAMP™, received U.S. Food and Drug Administration (FDA) clearance in July 2017. The first-of-its-kind, disposable laparoscopic surgical clamp offers technical and clinical improvement to surgeons performing laparoscopic sleeve gastrectomy, the most common and effective weight loss surgery.
"As a practicing Bariatric Surgeon and Founder of Standard Bariatrics, I recognized the variability in Sleeve Gastrectomy techniques and outcomes due to the limitations of the current surgical tools we use," said Jonathan Thompson, MD Founder, and Chief Medical Officer of Standard Bariatrics. "Therefore, our company is developing new and innovative products that can improve the laparoscopic resection of the stomach."
Standard Bariatrics was able to launch and grow in Southwest Ohio with seed investment from a vibrant startup ecosystem.
"Our seed financing, which was led by CincyTech and included Queen City Angels and Accelerant, was the vital capital that enabled Standard Bariatrics to demonstrate its value as an innovator with the launch of our first product," said Sokany.
###
About Standard Bariatrics, Inc.
Standard Bariatrics, Inc., is a Cincinnati based surgical procedure company focused on the innovative development and commercialization of medical devices. Driven by a passionate group of surgical innovators the company will continue to release surgical solutions designed to address significant market opportunities.. The Standard Bariatrics management team has over 175 combined years of experience in developing and commercializing novel medical device technologies with a record of achieving both clinical and economic excellence for patients and providers. For more information visit http://www.standardbariatrics.com or call 513-620-7751.
Media Contact
Matt Sokany
[email protected]
513-658-0328
@Cincy_Tech
http://www.cincytechusa.com