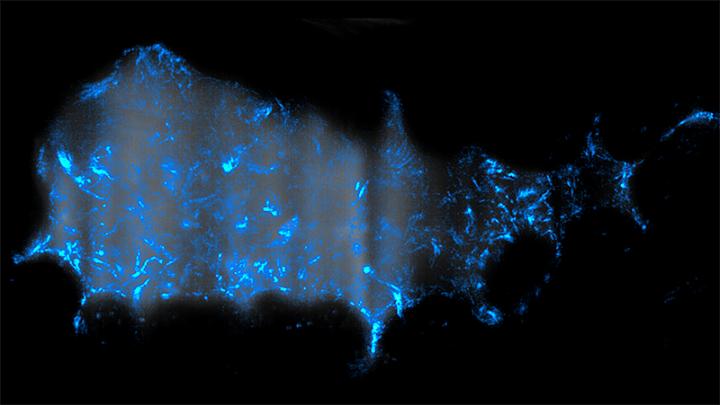
Credit: Image courtesy of Raghuveer Parthasarathy
EUGENE, Ore. — April 3, 2018 — Researchers experimenting with live zebrafish witnessed a 200-percent increase in the strength of intestinal contractions soon after exposure to the cholera-causing bacterium Vibrio cholerae, leading to expulsion of native gut bacteria.
The discovery, detailed in a paper online ahead of print in the Proceedings of the National Academy of Sciences, "was remarkable and unexpected," the eight co-authors wrote.
The researchers used genetic manipulation and cutting-edge three-dimensional microscopy to monitor what happens when the disease-causing microbe is initially introduced into zebrafish larvae, an organism commonly studied as a model for understanding health and disease in vertebrates, including humans.
The multidisciplinary team of physicists, molecular biologists and microbiologists focused on the harpoon-like injection capabilities of the type VI secretion system, an appendage found in many bacteria, including Vibrio cholerae. It transfers toxic proteins into competing healthy cells.
The scientists engineered Vibrio cholerae mutants with variations in that secretion system, and then observed the behavior of the microbes as they invaded zebrafish colonized with Aeromonas veronii, a native species in that animal's gut.
Instead of simply killing native Aeromonas gut bacteria upon contact, as expected, when Vibrio cholerae entered the gut the native bacteria were swiftly flushed out.
"The secretion system induced dramatic increases in the strength of the peristalsis process, the contractions that move gut contents down the gastrointestinal tract much like squeezing a tube of toothpaste from the end to the top," said study co-author Brian K. Hammer, a microbiologist at the Georgia Institute of Technology.
The researchers theorized that the unexpected bacterial manipulation in the digestive system might be driven by a particular piece of the type VI machinery known to bind to actin, a cellular scaffolding protein. The scientists deleted the actin-binding domain from the bacterial gene and saw that Vibrio cholerae lost its ability to enhance peristalsis and its ability to expel native Aeromonas.
The findings shed new light on how waterborne Vibrio cholerae functions. According to the Centers for Disease Control and Prevention, Vibrio cholerae triggers more than three million cases of acute diarrheal illness and 100,000 deaths in people worldwide each year.
"Knowing the strategies by which the bacterium is able to invade the intestine can open doors to therapies that might disrupt these paths," said corresponding author Raghuveer Parthasarathy, a professor of physics at the University of Oregon, whose imaging and analysis techniques were used in the study.
Because the type VI secretion system also is found in native gut bacteria, including those in the human gut microbiome, it potentially could be harnessed for therapies, including specially designed probiotics, to promote beneficial species or to defend against disease invasion, said Georgia Tech's Hammer.
"We suspect that other gut microbes, both pathogenic and beneficial, might similarly make use of this secretion system to reshape their environment," Parthasarathy said.
Most previous research on this secretion system has relied on studying bacteria outside of animals, on a Petri dish for example, or by examining fecal samples to infer what is happening in the gut during infection.
While the research team captured the impact of invasion by Vibrio cholerae, understanding just how it takes root in the host, such as what specific cells in the animal are targeted, is an open question, Parthasarathy said.
"We still have no idea how the action of this secretion system's harpoon is causing the changes in the muscle contractions," Hammer said. "We suspect that what we are observing may be an immune response to irritation in the gut lining, but what cells in the gut are being poked?"
How the findings may reflect the colonization of Vibrio cholerae in humans is not known, but the role of the secretion system makes a similar result plausible, the researchers wrote in their conclusion.
The findings emerged from a collaboration born in 2015 when Hammer, Parthasarathy and co-author Joao Xavier, a researcher at the Memorial Sloan-Kettering Cancer Center, discussed joint research possibilities during a conference, Scialog: Molecules Come to Life, in Tucson, Arizona.
The Scialog (Science and Dialog) was organized by the Research Corporation for Science Advancement and sponsored jointly with the Gordon and Betty Moore Foundation with additional support from the Simons Foundation. The goal of Scialog is to rapidly catalyze new interdisciplinary collaborative teams, such as the one formed by Hammer, Parthasarathy and Xavier, to work on high risk, high reward projects which emerge from the dialog.
As a result, their three labs received awards from the Gordon and Betty Moore Foundation and the Simons Foundation to pursue their Scialog idea. The National Science Foundation, National Institutes of Health, M.J. Murdock Charitable Trust and Kavli Microbiome Ideas Challenge also supported the research.
###
Media Contacts: Jim Barlow, director of science and research communications, 541-346-3481, [email protected], and A. Maureen Rouhi, director of communications, Georgia Tech College of Sciences, 404-385-5207, [email protected]
Sources: Raghuveer Parthasarathy, Alec & Kay Keith Professor of Physics, University of Oregon, 541-346-2933, [email protected], and Brian Hammer, associate professor, Biological Sciences, Georgia Tech, 404-385-7701, [email protected]; Joao Xavier, associate faculty member, Computational & Systems Biology Program, Memorial Sloan Kettering Cancer Center, 646-888-3195; [email protected]
Note: The UO is equipped with an on-campus television studio with a point-of-origin Vyvx connection, which provides broadcast-quality video to networks worldwide via fiber optic network. There also is video access to satellite uplink and audio access to an ISDN codec for broadcast-quality radio interviews.
Links:
Paper DOI: 10.1073/pnas.1720133115
About Raghu Parthasarathy: https://physics.uoregon.edu/profile/raghu/
Parthasarathy Lab: http://pages.uoregon.edu/raghu/
About Brian Hammer: http://biosci.gatech.edu/people/brian-hammer
Hammer Lab: http://www.hammerlab.biology.gatech.edu/
About Joao Xavier: https://www.mskcc.org/research-areas/labs/joao-xavier
Xavier lab: https://xavierlab.org/
Original 2015 funding, UO story: https://around.uoregon.edu/content/uo-physicist-parthasarathy-part-potentially-high-impact-project
Original 2015 funding, Georgia Tech story: http://biosci.gatech.edu/news-events/news/112
Media Contact
Jim Barlow
[email protected]
541-346-3481
@UOregonNews
http://around.uoregon.edu
Related Journal Article
http://dx.doi.org/10.1073/pnas.1720133115