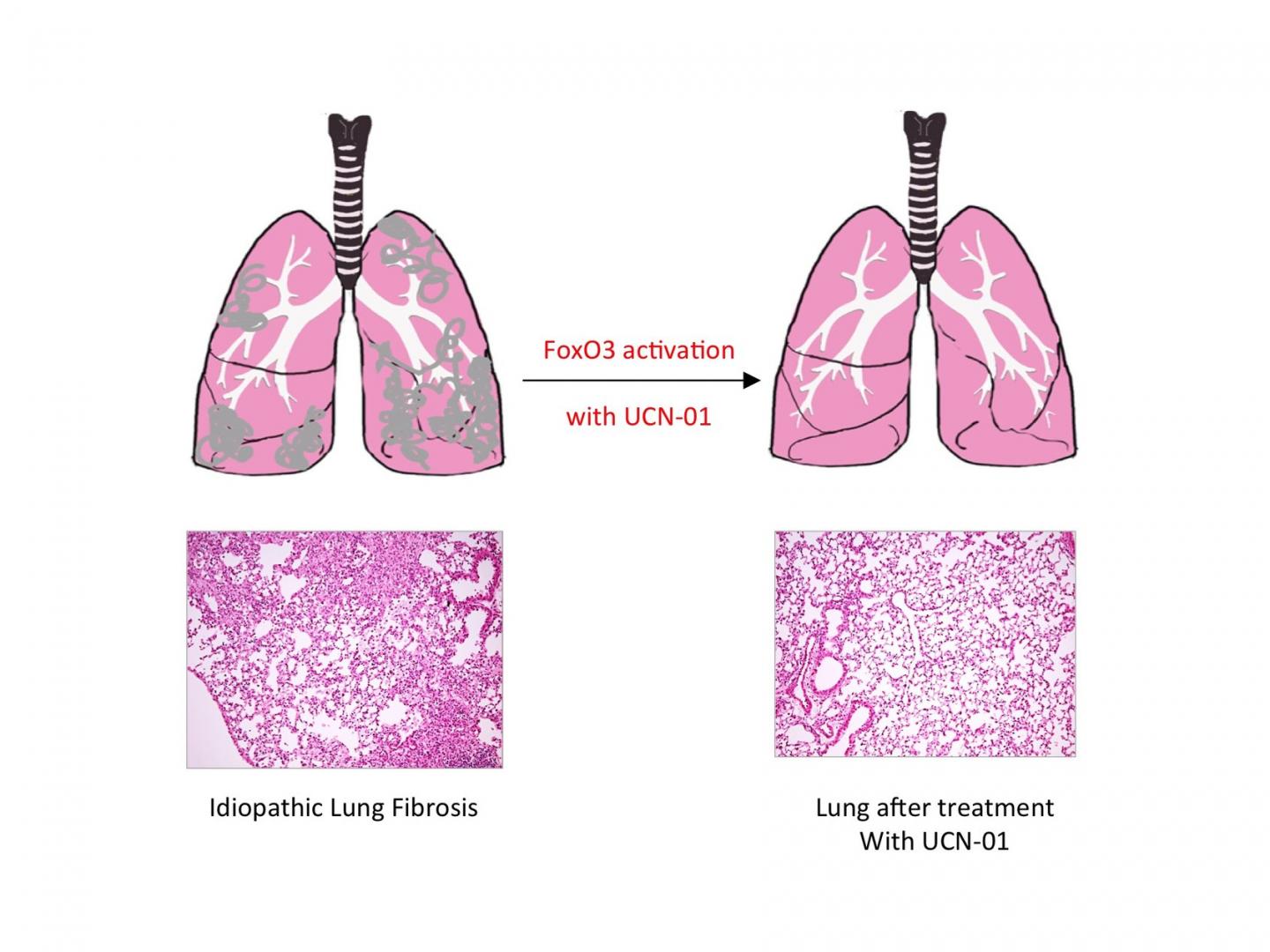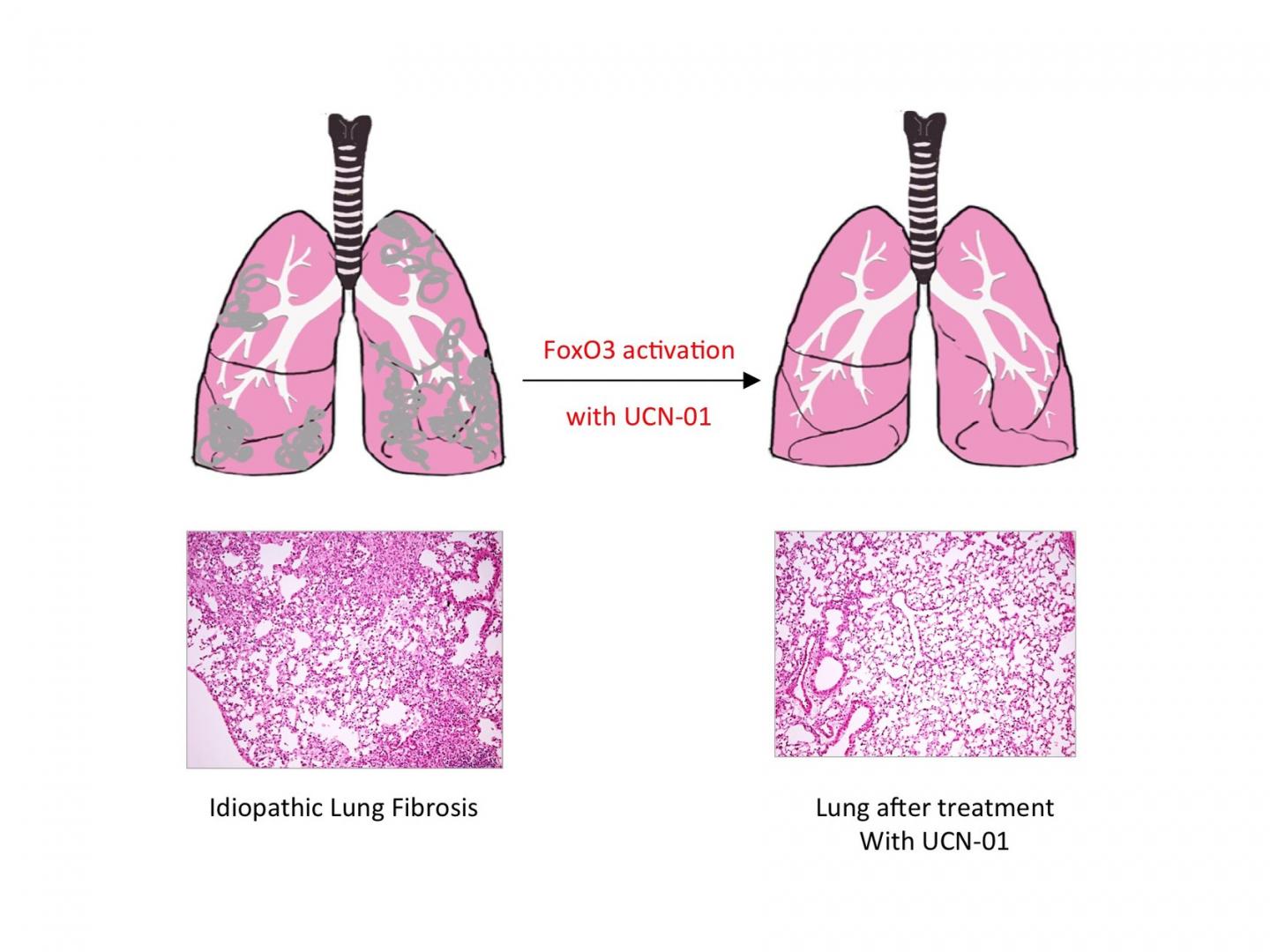
Credit: MPI f. Heart and Lung Research
To date, the molecular basis of pulmonary fibrosis has been poorly understood. Scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now shown that reduced activity of the transcription factor FoxO3 plays a key role in the development of the disease. In research on mice, the progress of the disease was able to be halted using drugs that boost FoxO3 activity. The researchers are hoping they may have found a possible approach to treatment.
Idiopathic pulmonary fibrosis is currently an incurable lung disease, in which sufferers lose the ability to absorb adequate oxygen. Although the word 'idiopathic' means that the cause is unknown, the disease primarily affects former and active heavy smokers from the age of 50.
An important role in idiopathic pulmonary fibrosis is played by connective tissue cells called fibroblasts. These cells provide structure to the air sacs (alveoli) in the lungs. During development of the disease, characteristic changes to these fibroblasts are observed. "The fibroblasts undergo a kind of personality change. In patients with pulmonary fibrosis, these cells contain increased amounts of contractile proteins, like those involved in muscle cell function," explains Soni Pullamsetti, who leads a Research Group at the Max Planck Institute for Heart and Lung Research. These modified cells, known as myofibroblasts, are responsible for the changes in connective tissue structure. As the disease progresses, the air sacs increasingly degenerate, resulting in damage to the blood vessels in the lungs. This results in shortness of breath.
Reduced FoxO3-activity
The researchers in Bad Nauheim decided to look for a factor which might be responsible for the fibroblast changes. Such a factor could hold the key to a possible treatment. Pullamsetti and her team first compared connective tissue cells from healthy individuals and patients with pulmonary fibrosis. "We noticed a transcription factor called FoxO3. Cells from patients with pulmonary fibrosis contained less of this protein than cells from healthy controls. The results were even clearer once we looked at FoxO3 activity – it was much lower in fibroblasts from patients with pulmonary fibrosis than in cells from healthy people," explains Pullamsetti.
In the light of this finding, the Max Planck researchers turned their attention to animal research and develped a mouse model of the disease. They found that mice with pulmonary fibrosis also had reduced FoxO3 activity. The effect was much greater in mice which had been genetically modified to lack FoxO3. "These mice developed idiopathic pulmonary fibrosis much more quickly than control animals – so quickly in fact that we were forced to shorten the experiment," explains Pullamsetti.
Affected mice respond to treatment
Reactivating FoxO3 in patients with pulmonary fibrosis might, therefore, offer a way of treating the disease. This approach proved successful in mice: treating mice with pulmonary fibrosis with UCN-01 resulted in a reduction in symptoms and improved lung function. This effect was not observed in mice that lacked FoxO3. UCN-01 is a substance which activates FoxO3 and is currently undergoing clinical trials as a tumour therapy.
"Our study shows that reduced FoxO3 activity plays an important role in the development of idiopathic pulmonary fibrosis, and that FoxO3 is a good place to start in developing a treatment for the disease," says Seeger. Further studies will examine this link more closely, in the hope of eventually being able to start trials on patients.
###
Original publication
H.M. Al-Tamari, S. Dabral, A. Schmall, P. Sarvari, C. Ruppert, J. Paik, R.A. DePinho, F. Grimminger, O. Eickelberg, A. Guenther, W. Seeger, R. Savai, S.S. Pullamsetti
FoxO3 an important player in fibrogenesis and therapeutic target for idiopathic pulmonary fibrosis.
EMBO Mol. Med.; December 7, 2017
Media Contact
Dr. Werner Seeger
[email protected]
49-603-270-5380
@maxplanckpress
http://www.mpg.de
Original Source
https://www.mpg.de/11868863/pulmonary-fibrosis-foxo3 http://dx.doi.org/10.15252/emmm.201606261