How do stem cells preserve their ability to become any type of cell in the body? And how do they “decide” to give up that magical state and start specializing?
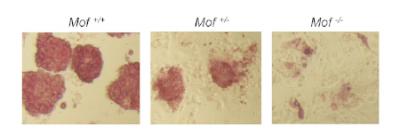
Mouse stem cells with two normally functioning copies of the Mof gene (left) have intact “stem-ness” — but that ability to self-renew is lost in cells in which one or both copies of Mof don’t work correctly (middle and right). Photo Credit: Dou laboratory, University of Michigan
If researchers could answer these questions, our ability to harness stem cells to treat disease could explode. Now, a University of Michigan Medical School team has published a key discovery that could help that goal become reality.
In the current issue of the prestigious journal Cell Stem Cell, researcher Yali Dou, Ph.D., and her team show the crucial role of a protein called Mof in preserving the ‘stem-ness’ of stem cells, and priming them to become specialized cells in mice.
Their results show that Mof plays a key role in the “epigenetics” of stem cells — that is, helping stem cells read and use their DNA. One of the key questions in stem cell research is what keeps stem cells in a kind of eternal youth, and then allows them to start “growing up” to be a specific type of tissue.
Dou, an associate professor of pathology and biological chemistry, has studied Mof for several years, puzzling over the intricacies of its role in stem cell biology.
She and her team have zeroed in on the factors that add temporary tags to DNA when it’s coiled around tiny spools called histones. In order to read their DNA, cells have to unwind it a bit from those spools, allowing the gene-reading mechanisms to get access to the genetic code and transcribe it. The temporary tags added by Mof act as tiny beacons, guiding the “reader” mechanism to the right place.
“Simply put, Mof regulates the core transcription mechanism – without it you can’t be a stem cell,” says Dou. “There are many such proteins, called histone acetyltransferases, in cells – but only MOF is important in undifferentiated cells.”
Dou and her team also have published on another protein involved in DNA transcription, called WDR5, that places tags that are important during transcription. But Mof appears to control the process that actually allows cells to determine which genes it wants to read – a crucial function for stem-ness. “Without Mof, embryonic stem cells lost their self-renewal capability and started to differentiate,” she explains.
The new findings may have particular importance for work on induced pluripotent stem cells – the kind of stem cells that don’t come from an embryo, but are made from “adult” tissue.
IPCS research holds great promise for disease treatment because it could allow a patient to be treated with stem cells made from their own tissue. But the current way of making IPSCs from tissue involves a process that uses a cancer-causing gene – a step that might give doctors and patients pause.
Dou says that further work on Mof might make it possible to stop using that potentially harmful approach. But further research will be needed.
What they will focus on is how Mof marks the DNA structures called chromatin to keep parts of the genome readily accessible. In stem cells, scientists have shown, many areas of DNA are kept open for access – probably because stem cells need to use their DNA to make many proteins that keep them from ‘growing up.’
Once a stem cell starts to differentiate, or become a certain specialized type of cell, parts of the DNA close up and aren’t as accessible. Many scientific teams have studied this “selective silencing” and the factors that cause stem cells to start specializing by reading only certain genes. But few have looked at the factors that facilitate broad-range DNA transcription to preserve stem-ness.
“Mof marks the areas that need to stay open and maintains the potential to become anything,” Dou explains. Its crucial role in many species is hinted at by the fact that the gene to make Mof has the same sequence in fruit flies and mice.
“If you think about stem cell biology, the self-renewal is one aspect that makes stem cells unique and powerful, and the differentiation is another,” says Dou. “People have looked a lot at differentiation to make cells useful for therapy in the future – but the stem cell itself is actually pretty fascinating. So far, Mof is the only histone acetyltransferase found to support the stemness of embryonic stem cells.”
Story Source:
The above story is based on materials provided by University of Michigan Medical School.