Researchers at Osaka University, Japan uncovered the mechanisms that suppress the propagation of the hepatitis C virus (HCV) with the potential of improving pathological liver conditions. Using model mice, they confirmed that when a certain enzyme is inhibited, HCV particle production is reduced leading to an improvement of pathological liver conditions. They thereby identified a new drug target for the development of new HCV drugs.
About 200 million people around the world are infected with the HCV virus. HCV infection may cause fatty liver, hepatic fibrosis and liver cancer. In Japan, the HCV virus is the main cause for viral liver cancer, constituting 70% of liver cancers. Although the recent development of effective drugs targeting HCV replicative enzymes has enabled the elimination of HCV, challenges remain including the emergence of resistant viruses and the development of liver cancer after virus elimination. So far it was known that the cleavage of the HCV core protein by the enzyme signal-peptide peptidase (SPP) in infected host cells played an important role in the formation of viral particles and the development of pathological liver conditions. However, the details of this mechanism were not understood.
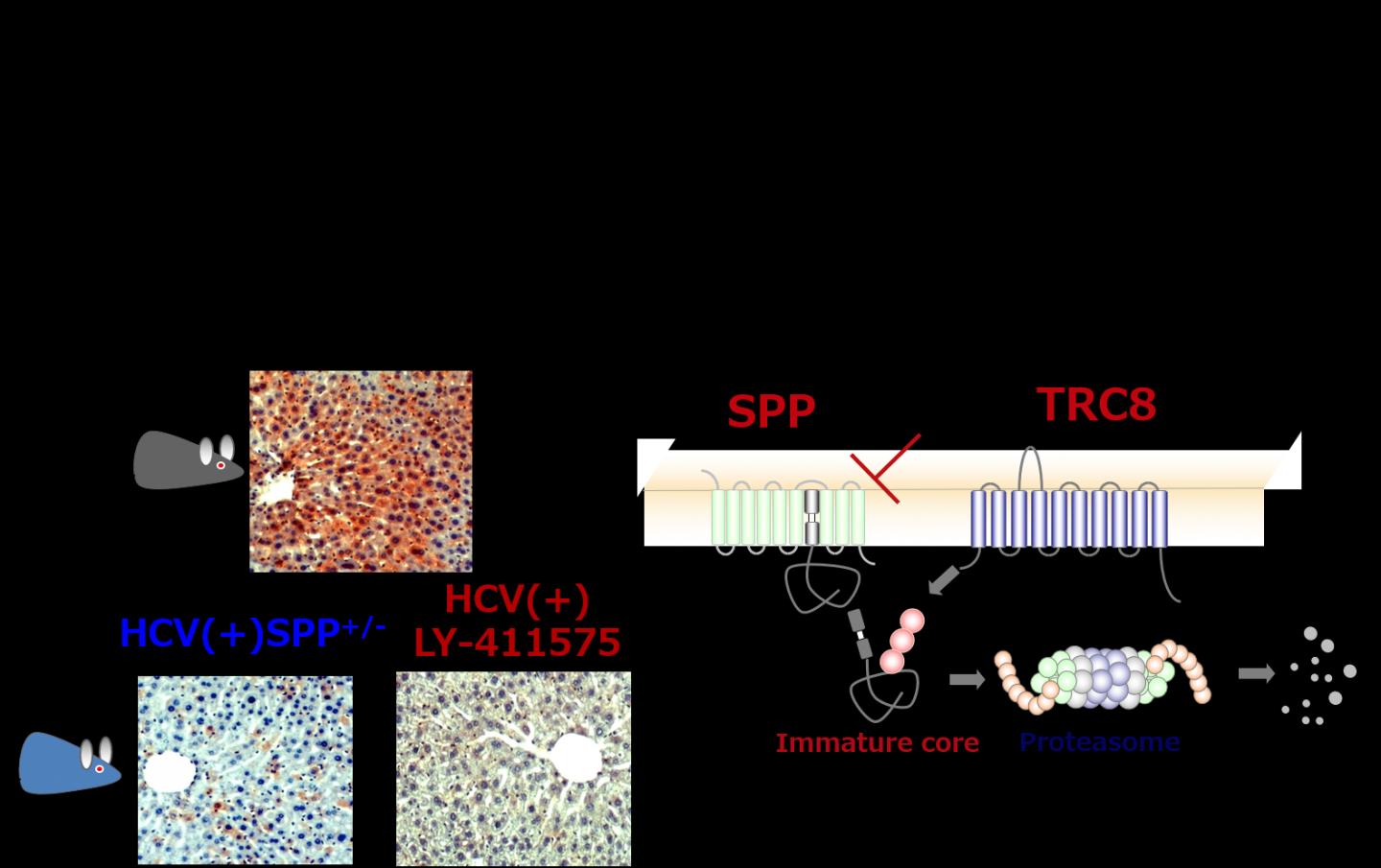
A research group led by Toru Okamoto, assistant professor and Yoshiharu Matsuura, professor at Research Institute for Microbial Diseases, Osaka University has now discovered that when the enzyme SPP is inhibited, HCV particle production is reduced resulting in an improvement of pathological liver conditions.
The researchers found a chemical compound that inhibits the SPP enzyme in the y-secretase inhibitor which is currently in the development process for Alzheimer's disease treatment. They also discovered that the immature core proteins which are not cleaved by SPP are recognized by the enzyme TRC8 and quickly degraded. If this degradation process is suppressed, cellular damage is strongly induced by endoplasmic reticulum stress (ER stress). The endoplasmic reticulum is central to protein biosynthesis and in a state of ER stress, the proteins synthesizing there are unable to fold up correctly thereby causing cell damage. This degradation process can therefore be considered as a quality control mechanism for new proteins. When the researchers administered the SPP inhibitor to model mice, HCV particle production was significantly reduced, improving HVC pathologic conditions such as insulin resistance and fatty liver.
The results of this study suggest the development of SPP inhibitors as a new hepatitis C drug. In addition, the observed protein quality control mechanism via SPP/TRC8 is thought to be related to other diseases as well thereby being potentially useful for the drug development for a variety of diseases.
###
This research was featured in the electronic version of Nature Communications on Wednesday, May 4, 2016 (British Time).
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
@osaka_univ_e
http://www.osaka-u.ac.jp/en





