Credit: Dr. Bimal Banik et al., Bentham Science Publishers
Indoloquinoline alkaloids have received significant popularity because of their antimalarial and DNA intercalating properties as well as their use as folk medicines for the control of fever, amoebiasis and malaria. Related metabolites include cryptotackieine and norcryptotackieine which are linear indolo[2,3-b]quinoline alkaloids. These molecules have demonstrated numerous medicinal activities including antimicrobial, and cytotoxicity. They act through DNA intercalation and inhibition of topoisomerase II inhibition. Consequently, organic and medicinal chemists and biologists have become interested in their concise preparation and studies of their biological activities. A few synthetic routes have been developed for the preparation of these structurally unique and complex indole heterocycles. Interestingly, most of these available methods are one-pot methods. For example, high temperature reaction of molecular iodine-catalyzed reaction in diphenyl ether, pivalic acid and ruthenium-exchanged FAU-Y zeolite in 1,4-dioxane is performed. N-Bromosuccinimide at room temperature is found to be good for this purpose. The principal limitation is that almost all of these methods require a very long time. Moreover, the yields of the target molecules are low.
The current research is aimed to develop an efficient synthetic method for the synthesis of indoloquinoline alkaloids mentioned above. Considering the known procedures and analyzing the conditions of the experiments, the iodine-catalyzed tandem method is proved to be excellent. Despite the superiority of iodine, this method produces products with low to moderate yields, requires long reaction time and high temperature. Therefore, an improvement of molecular iodine-catalyzed reaction for this purpose is necessary.
Our research group has been exploring microwave-induced reactions for more than 25 years. Reactions conducted under microwave oven are known to be rapid and successful at much less drastic conditions. Moreover, they also produce the target molecules in better yields without giving side reactions in most of the examples. In conventional methods, unwanted side reactions in many instances lower the yields of the products or produce mixture of products and therefore, isolation of the desired compounds become tedious. On the basis of several successes on microwave-induced chemistry and our interests in the preparation of medicinally useful compounds, we have investigated the preparation of cryptotackieine and norcryptotackieine and their derivatives in automated microwave oven in tightly sealed reaction container.
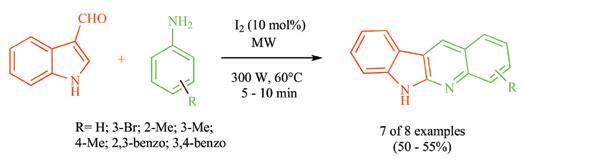
Indole-3-carboxyaldehyde and two equivalents of aniline in presence of 10 mol% of molecular iodine at 600C and 300 watt power in a sealed vessel are irradiated in an automated microwave. It is interesting to note that the reaction proceeds rapidly with an increase in the yield of the product compared to the conventional heating (oil bath or heating mantle) at much higher temperature. The structure of the starting reactants and products suggests a most probable mechanism. Dehydration to imine from aldehyde and amine, nucleophilic reaction of the imine, annulation and subsequent aromatization occurs in a tandem process in a one-pot method.
This method is extended for the preparation of 6H-indolo[2,3-b]quinolines. As expected, the previously known compounds are synthesized very rapidly and with simple work-up, and without extensive purification. No solvent is used when one of the reactants is liquid or oil. These reactions are also performed in the presence of solvents (THF, MeOH, CHCl3, and CH2Cl2). THF and MeOH are proved to be the best solvent whereas CHCl3 and CH2Cl2 are moderately good for this method.
An expeditious simple and rapid microwave-assisted procedure for the synthesis of significant types of indoloquinolines using molecular iodine as catalyst in a one-pot is developed. The procedure described herein is the first rapid method for the synthesis of novel indoloquinolines. The rate acceleration by microwave-induced method is probably due to the alteration of the transition state of the series of reactions that occur in this tandem process. The benefit of this process is the lowered energy consumption compared to traditional method under reflux or high temperature that requires input of latent heat of vaporization. In this method, vaporization is not possible and so little expenditure of latent heat occurs. The non-ionizing radiation of the microwave is absorbed very efficiently by the reactants and polar solvents and thereby accelerates the process in an unbelievable way.
###
Prakash T. Parvatkara,b, P. S. Parameswaranc, Debasish Bandyopadhyayd, Sanghamitra Mukherjeed, Bimal K. Banikd,e*
aDepartment of Chemistry, Goa University, Taleigao Plateau, Goa 403 206, India;
bCurrent Address: Department of Chemistry and Chemical Biology, Northeastern University, Boston, MA 02115, United States
cCSIR-National Institute of Oceanography, Regional Centre, Kochi 682 018, India
dDepartment of Chemistry, The University of Texas-Pan American, Edinburg, TX 78539, United States; eCurrent Address: Community Health System of South Texas, 3135 South Sugar Road, Edinburg, TX 78539, United States; Phone: 281-813-2104, Fax: 956-259-8085; Email: [email protected]; [email protected]
Media Contact
Faizan ul Haq
[email protected]
@BenthamScienceP
http://benthamscience.com/
Related Journal Article
http://dx.doi.org/10.2174/2213335604666170412162854