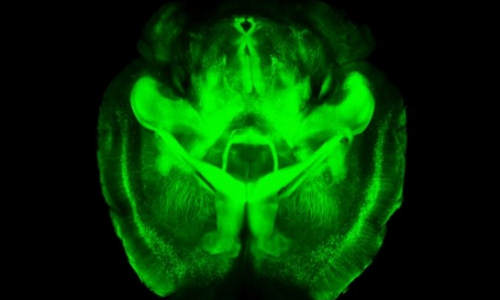
Last year Karl Deisseroth, a Stanford professor of bioengineering and of psychiatry and behavioral sciences, announced a new way of peering into a brain – removed from the body – that provided spectacular fly-through views of its inner connections. Since then laboratories around the world have begun using the technique, called CLARITY, with some success, to better understand the brain’s wiring.
A three-dimensional rendering of clarified brain imaged from below. Photo Credit: Deisseroth lab
However, Deisseroth said that with two technological fixes CLARITY could be even more broadly adopted. The first problem was that laboratories were not set up to reliably carry out the CLARITY process. Second, the most commonly available microscopy methods were not designed to image the whole transparent brain. “There have been a number of remarkable results described using CLARITY,” Deisseroth said, “but we needed to address these two distinct challenges to make the technology easier to use.”
In a Nature Protocols paper published June 19, Deisseroth presented solutions to both of those bottlenecks. “These transform CLARITY, making the overall process much easier and the data collection much faster,” he said. He and his co-authors, including postdoctoral fellows Raju Tomer and Li Ye and graduate student Brian Hsueh, anticipate that even more scientists will now be able to take advantage of the technique to better understand the brain at a fundamental level, and also to probe the origins of brain diseases.
This paper may be the first to be published with support of the White House BRAIN Initiative, announced last year with the ambitious goal of mapping the brain’s trillions of nerve connections and understanding how signals zip through those interconnected cells to control our thoughts, memories, movement and everything else that makes us us.
“This work shares the spirit of the BRAIN Initiative goal of building new technologies to understand the brain – including the human brain,” said Deisseroth, who is also a Stanford Bio-X affiliated faculty member.
Eliminating fat
When you look at the brain, what you see is the fatty outer covering of the nerve cells within, which blocks microscopes from taking images of the intricate connections between deep brain cells. The idea behind CLARITY was to eliminate that fatty covering while keeping the brain intact, complete with all its intricate inner wiring.
The way Deisseroth and his team eliminated the fat was to build a gel within the intact brain that held all the structures and proteins in place. They then used an electric field to pull out the fat layer that had been dissolved in an electrically charged detergent, leaving behind all the brain’s structures embedded in the firm water-based gel, or hydrogel. This is called electrophoretic CLARITY.
The electric field aspect was a challenge for some labs. “About half the people who tried it got it working right away,” Deisseroth said, “but others had problems with the voltage damaging tissue.” Deisseroth said that this kind of challenge is normal when introducing new technologies. When he first introduced optogenetics, which allows scientists to control individual nerves using light, a similar proportion of labs were not initially set up to easily implement the new technology, and ran into challenges.
To help expand the use of CLARITY, the team devised an alternate way of pulling out the fat from the hydrogel-embedded brain – a technique they call passive CLARITY. It takes a little longer, but still removes all the fat, is much easier and does not pose a risk to the tissue. “Electrophoretic CLARITY is important for cases where speed is critical, and for some tissues,” said Deisseroth, who is also the D.H. Chen Professor. “But passive CLARITY is a crucial advance for the community, especially for neuroscience.” Passive CLARITY requires nothing more than some chemicals, a warm bath and time.
Many groups have begun to apply CLARITY to probe brains donated from people who had diseases like epilepsy or autism, which might have left clues in the brain to help scientists understand and eventually treat the disease. But scientists, including Deisseroth, had been wary of trying electrophoretic CLARTY on these valuable clinical samples with even a very low risk of damage. “It’s a rare and precious donated sample, you don’t want to have a chance of damage or error,” Deisseroth said. “Now the risk issue is addressed, and on top of that you can get the data very rapidly.”
Fast CLARITY imaging in color
The second advance had to do this rapidity of data collection. In studying any cells, scientists often make use of probes that will go into the cell or tissue, latch onto a particular molecule, then glow green, blue, yellow or other colors in response to particular wavelengths of light. This is what produces the colorful cellular images that are so common in biology research. Using CLARITY, these colorful structures become visible throughout the entire brain, since no fat remains to block the light.
But here’s the hitch. Those probes stop working, or get bleached, after they’ve been exposed to too much light. That’s fine if a scientist is just taking a picture of a small cellular structure, which takes little time. But to get a high-resolution image of an entire brain, the whole tissue is bathed in light throughout the time it takes to image it point by point. This approach bleaches out the probes before the entire brain can be imaged at high resolution.
The second advance of the new paper addresses this issue, making it easier to image the entire brain without bleaching the probes. “We can now scan an entire plane at one time instead of a point,” Deisseroth said. “That buys you a couple orders of magnitude of time, and also efficiently delivers light only to where the imaging is happening.” The technique is called light sheet microscopy and has been around for a while, but previously didn’t have high enough resolution to see the fine details of cellular structures. “We advanced traditional light sheet microscopy for CLARITY, and can now see fine wiring structures deep within an intact adult brain,” Deisseroth said. His lab built their own microscope, but the procedures are described in the paper, and the key components are commercially available. Additionally, Deisseroth’s lab provides free training courses in CLARITY, modeled after his optogenetics courses, to help disseminate the techniques.
Brain imaging to help soldiers
The BRAIN Initiative is being funded through several government agencies including the Defense Advanced Research Projects Agency (DARPA), which funded Deisseroth’s work through its new Neuro-FAST program. Deisseroth said that like the National Institute of Mental Health (NIMH, another major funder of the new paper), DARPA “is interested in deepening our understanding of brain circuits in intact and injured brains to inform the development of better therapies.” The new methods Deisseroth and his team developed will accelerate both human- and animal-model CLARITY; as CLARITY becomes more widely used, it will continue to help reveal how those inner circuits are structured in normal and diseased brains, and perhaps point to possible therapies.
Other arms of the BRAIN Initiative are funded through the National Science Foundation (NSF) and the National Institutes of Health (NIH). A working group for the NIH arm was co-led by William Newsome, professor of neurobiology and director of the Stanford Neurosciences Institute, and also included Deisseroth and Mark Schnitzer, associate professor of biology and of applied physics. That group recently recommended a $4.5 billion investment in the BRAIN Initiative over the next 12 years, which NIH Director Francis Collins approved earlier this month.
Story Source:
The above story is based on materials provided by Stanford University, Amy Adams.