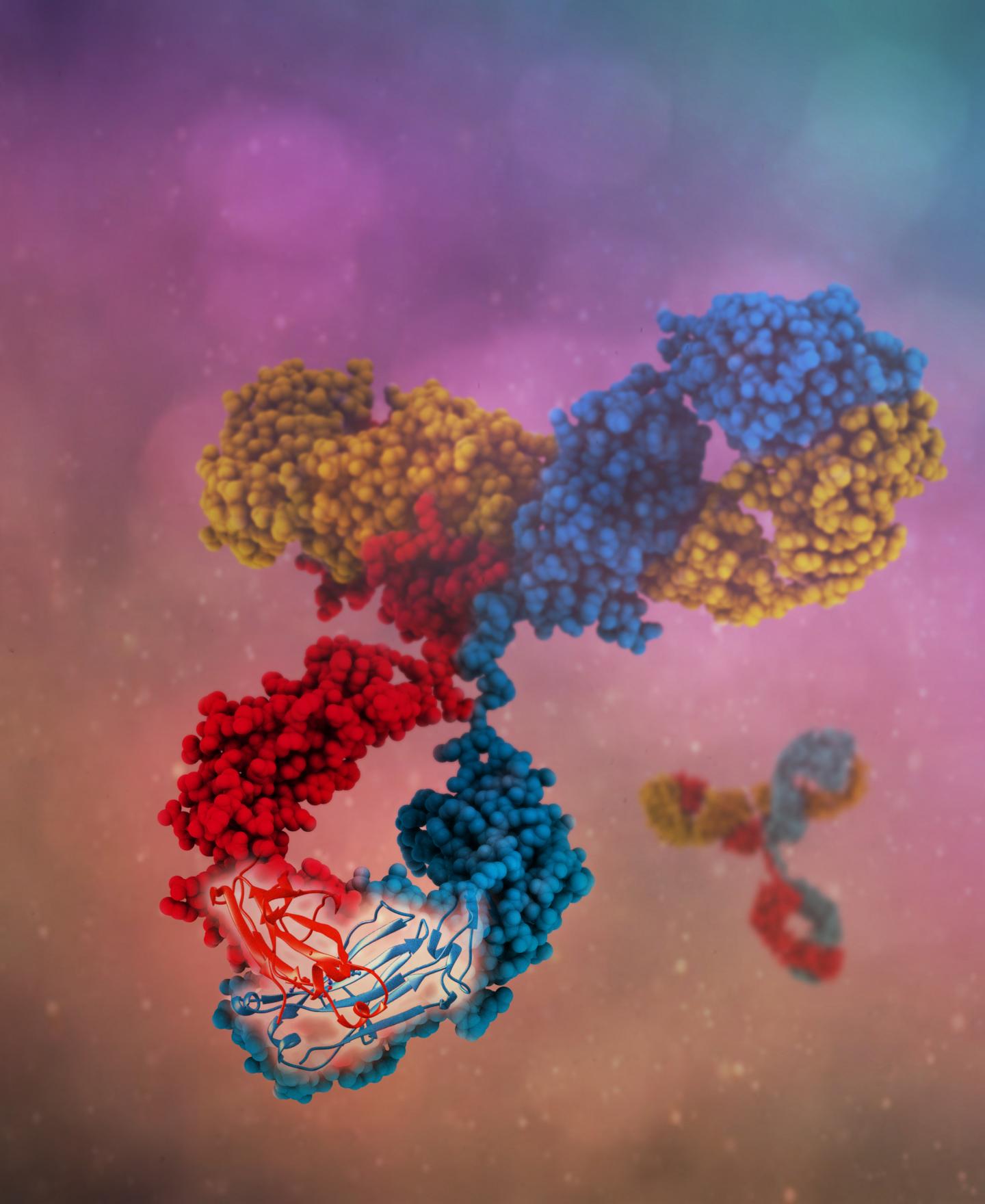
Credit: Camilla De Nardis, Linda J. A. Hendriks, Emilie Poirier, Tudor Arvinte, Piet Gros, Alexander B. H. Bakker and John de Kruif
A group of researchers has developed an approach to efficiently produce antibodies that can bind to two different target molecules simultaneously, a long-desired innovation in the field of cancer immunotherapy. The details will be published in the Sept. 1 issue of the Journal of Biological Chemistry.
Antibodies are proteins produced by the immune system that specialize in recognizing and binding to molecular targets unique to bacteria, viruses or other foreign cells. Because antibodies are stable and long-lasting in the human body and can precisely recognize specific targets, they have been exploited to develop new treatments for diseases. For example, modified antibodies can be used to bind to targets in cancer cells, recruiting the immune system to attack the cancer or preventing the cancer cells from multiplying. Because of their precision and capacity to stimulate the body's immune response, antibody-based therapies typically have fewer side effects than chemotherapy or radiation.
Antibodies are "Y" shaped, and typically bind a target, or antigen, through the tip of each arm of the "Y." In naturally produced antibodies, both arms of a single antibody typically are the same and bind to the same target. One approach to increasing the versatility of antibody therapies is to engineer what are called bispecific antibodies, in which each arm binds to a different molecule. This expands the range of what antibodies can be used for. For example, a bispecific antibody could target a cluster of proteins made up of multiple protein types, or it could bring two different molecules or cell types together.
One bispecific antibody-like drug – the leukemia drug blinatumomab – is currently on the market. But development of more therapies based on bispecific antibodies has been hampered by technical challenges. For example, certain bispecific antibodies deviate from the standard Y shape and tend to be less stable than conventional antibodies, falling apart easily. Further, certain bispecific antibody formats have tended to be difficult to produce at industrial scales because they can require specialized engineering processes.
In experiments published in JBC, a team overseen by John de Kruif, the chief technology officer of clinical-stage research company Merus N.V., engineered improved bispecific antibodies by making a few key changes to the structure of natural human immunoglobulin G (IgG) antibodies, and showed that they could be readily manufactured. IgG is a well-studied antibody and is the most abundant one produced in the human body.
"We have made, in a complete IgG molecule, only four changes to get from a normal monospecific antibody into bispecific antibody," de Kruif said. "The great thing is that it looks so much like a normal antibody that we can produce it well and we believe we know how it will behave."
The four mutations were in the "heavy chain" protein components of the antibodies. Typically, two identical heavy chains pair up in each antibody. The challenge in creating bispecific antibodies was to induce non-identical heavy chains to pair–creating "arms" capable of binding different antigens – while discouraging pairing of identical ones.
The team's idea was to introduce amino acids with opposite charges to the two different heavy chains, so that identical heavy chains would repel each other whereas the positively and negatively charged heavy chains would attract. To identify the right locations in which to introduce these charges, they used computational simulations using virtual screening software followed by validation in the lab.
"Using the virtual screening software provided a baseline," said Linda Kaldenberg-Hendriks of Merus, who led the testing of the antibodies. "We identified potential good candidates for design choices in the heavy chain sets, then generated the proteins and characterized them thoroughly. When we saw that they were behaving the way we wanted them to, it was really satisfying."
The team also investigated the molecular structure of the bispecific antibodies, and confirmed that the mutations resulted in only very subtle changes in the "backbone" of the heavy chains, which may explain stability of these bispecific antibodies.
"A strong point [of this study] was to combine different approaches, the computational tools with the biochemistry and structural biology," said Camilla De Nardis of Merus and Utrecht University, who was a co-lead author on the study.
The proteins that worked paired up to form bispecific antibodies, with very few to no monospecific antibodies in the mixture. The team next subjected them to a battery of tests, confirming that they were as stable as normal IgG antibodies and had similar pharmacokinetic properties.
Because production and purification of IgG antibodies is a well-established industrial process, the team could simply provide manufacturers with the protein sequences modified with the key changes that allowed the proteins to form bispecifics. "We believe we can make virtually any bispecific antibody we want," Kaldenberg-Hendriks said.
The team's bispecific antibodies targeting cancer cell growth factor complexes are now in clinical trials, with more still in the preclinical pipeline. The team is enthusiastic about the potential for the versatile format to be adapted to different types of therapies.
"Antibodies are capable of being so specific, and you can tweak and tune them," Kaldenberg-Hendriks said. "With bispecific antibodies, we believe we can choose the affinities of both arms and balance them so that you can more specifically target tumors, and also recruit other cells or molecules to attack the tumor cells without many side effects. We really think it's the way forward."
###
About the Journal of Biological Chemistry
JBC is a weekly peer-reviewed scientific journal that publishes research "motivated by biology, enabled by chemistry" across all areas of biochemistry and molecular biology.
About the American Society for Biochemistry and Molecular Biology
The ASBMB is a nonprofit scientific and educational organization with more than 12,000 members worldwide. Most members teach and conduct research at colleges and universities. Others conduct research in various government laboratories, at nonprofit research institutions and in industry. The Society's student members attend undergraduate or graduate institutions. For more information about ASBMB, visit http://www.asbmb.org.
The work was funded by BioStruct-X Grant 283570 embedded in the European Union's Seventh Framework Programme FP7/2007-2013.
Media Contact
Sasha Mushegian
[email protected]
@asbmb
http://www.asbmb.org
Related Journal Article
http://dx.doi.org/10.1074/jbc.M117.793497