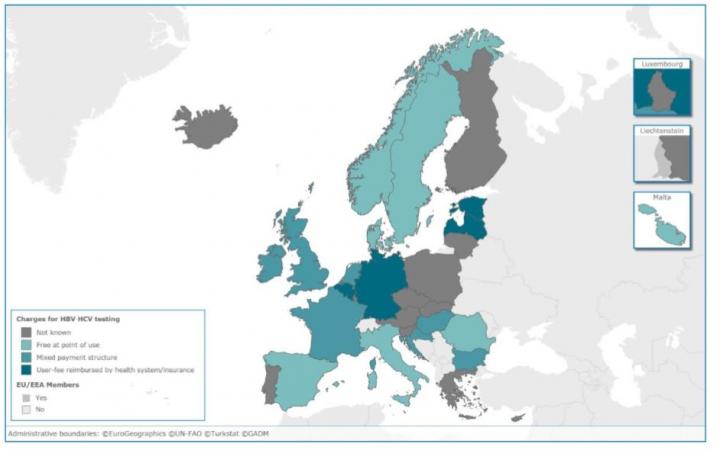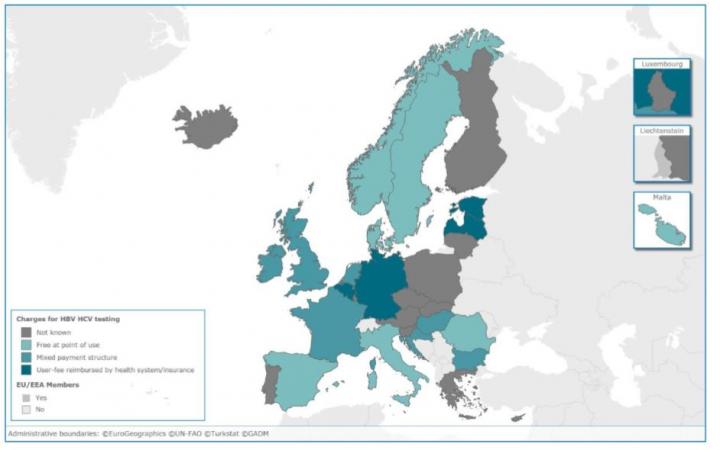
Credit: European Centre for Disease Prevention and Control, ECDC
The survey results suggest a wide variation in existing national testing policy and practice when it comes to hepatitis B and C – with overall limited monitoring of testing, diagnosis, and treatment across EU/EEA Member States. Many respondents expressed a need for Europe-wide practical guidance on how testing initiatives should be conducted, evaluated, and monitored.
An estimated 9 to 10 million Europeans have been infected with the hepatitis B (HBV) or hepatitis C virus (HCV) in the countries of the European Union and European Economic Area (EU/EEA), and many are unaware of their infection. ECDC undertook two surveys to evaluate needs and priorities to inform a Guidance on HBV/HCV testing and screening in the EU and to assess the availability of monitoring data on the HBV and HCV epidemic against the core indicators defined in the WHO Regional Action Plan to eliminate viral hepatitis by 2030. 20 (65%) and 21 (68%) Member States responded to the two surveys.
The results suggest a wide variation in HBV/HCV testing policy and practice across the EU/EEA showing significant gaps in available testing guidance and a lack of national monitoring programmes regarding all aspects of testing, diagnosis, treatment, morbidity, and mortality.
Less than half of the responding EU/EEA countries have dedicated HBV or HCV testing guidance in place (29% and 48% respectively), with the remainder having either a number of policy documents where HBV/HCV is included, or no testing policy at all. Just more than half of the countries had a policy on testing of HBV/HCV among people who inject drugs in place, while other key groups such as commercial sex workers, me who have sex with men and people receiving tattoos or piercings in unregulated settings were mostly omitted.
A need for European-level guidance
At the implementation level, more than three out of four responding countries reported that they felt that risk groups were not targeted effectively by existing testing programmes. Just over half of responding countries agreed that there is a need for a new European-level testing guidance, in particular guidance on who to test, how to target those at risk, and monitoring and evaluation of testing initiatives.
With regards to monitoring indicators, almost all the responding countries collected some morbidity and mortality data on hepatitis B and C's long term complication, liver cancer.
However data on the HBV or HCV status of the patient was recorded by only a few. The most frequently monitored indicator for HCV was the number of people undergoing treatment (12 countries), whereas the number of those treated for HBV is only monitored in seven countries.
What is more: there are no indicators for hepatitis B or C infection that are monitored routinely by all responding Member States, meaning that further work would be required before any meaningful EU-wide monitoring can be established.
This baseline assessment on the situation in the EU/EEA, despite its limitations due to its the response rate, provides invaluable information towards the development of a planned ECDC Guidance on hepatitis testing helping to ensure that this new Guidance will focus on the main gaps identified.
###
Read the full report Hepatitis B and C testing activities, needs, and priorities in the EU/EEA
Read more
Systematic review on hepatitis B and C prevalence in the EU/EEA
Annual Epidemiological Report:
Hepatitis B – 2015 data for the EU/EEA
Hepatitis C – 2015 data for The EU/EEA
Media Contact
ECDC Press Office
[email protected]
46-858-601-678
@ECDC_EU
http://ecdc.europa.eu
############
Story Source: Materials provided by Scienmag