Researchers at UC Berkeley found that an electrical current can be used to orchestrate the flow of a group of cells, an achievement that could establish the basis for more controlled forms of tissue engineering and for potential applications such as “smart bandages” that use electrical stimulation to help heal wounds.
Videos show the effect of electric fields on the movement of epithelial cells. The first clip shows the cells migrating normally until the electric field is turned on, causing the population to move right. In the second video, the electric field causes the population of cells to make a U-turn. (Video by Daniel Cohen)
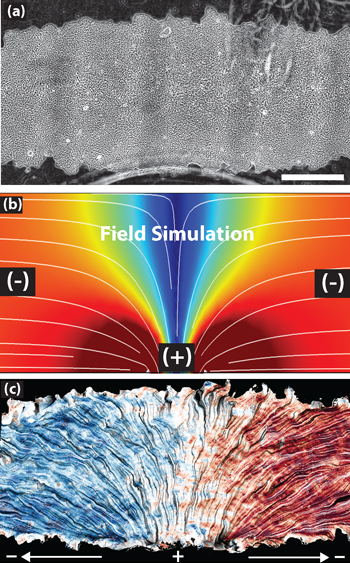
In the experiments, described in a study published this week in the journal Nature Materials, the researchers used single layers of epithelial cells, the type of cells that bind together to form robust sheathes in skin, kidneys, cornea and other organs. They found that by applying an electric current of about five volts per centimeter, they could encourage cells to migrate along the direct current electric field.
They were able to make the cells swarm left or right, to diverge or converge and to make collective U-turns. They also created elaborate shapes, such as a triceratops and the UC Berkeley Cal bear mascot, to explore how the population and configuration of cell sheets affect migration.
Directing herds vs. individuals
“This is the first data showing that direct current fields can be used to deliberately guide migration of a sheet of epithelial cells,” said study lead author Daniel Cohen, who did this work as a student in the UC Berkeley-UC San Francisco Joint Graduate Program in Bioengineering. “There are many natural systems whose properties and behaviors arise from interactions across large numbers of individual parts – sand dunes, flocks of birds, schools of fish, and even the cells in our tissues. Just as a few sheepdogs exert enormous control over the herding behavior of sheep, we might be able to similarly herd biological cells for tissue engineering.”
Galvanotaxis – the use of electricity to direct cell movement – had been previously demonstrated for individual cells, but how it influences the collective motion of cells was still unclear.
“The ability to govern the movement of a mass of cells has great utility as a scientific tool in tissue engineering,” said study senior author Michel Maharbiz, UC Berkeley associate professor of electrical engineering and computer sciences. “Instead of manipulating one cell at a time, we could develop a few simple design rules that would provide a global cue to control a collection of cells.”
The work was borne from a project, led by Maharbiz, to develop electronic nanomaterials for medical use that was funded by the National Science Foundation’s Emerging Frontiers in Research and Innovation program. The researchers collaborated with W. James Nelson, professor of molecular and cellular physiology at Stanford University and one of the world’s top experts in cell-to-cell adhesion. Cohen is now a postdoctoral research fellow in Nelson’s lab.
The top image shows a patch of epithelial cells. The white lines in the middle image mark the electric current flowing from positive to negative over the cells. The bottom image shows how the cells track the electric field, with blue indicating leftward migration and red signaling rightward movement. (Images by Daniel Cohen)
Possible wound healing applications
With our bodies full of flowing ions and salt solutions, it is no surprise that electrical signals play a big role in our physiology, from neural transmissions to muscle stimulation.
“The electrical phenomenon we are exploring is distinct in that the current produced is providing a cue for cells to migrate,” said Maharbiz.
The study authors are exploring the role of bioelectrical signals in the wound healing process, building upon the discovery in 1843 that an injury to the body creates a change in the electrical field at the wound site. By mapping the changes in the electrical field when an injury occurs and as it heals, the researchers may be able to develop technology to help speed and improve the repair process.
“These data clearly demonstrate that the kind of cellular control we would need for a smart bandage might be possible, and the next part of our work will focus on adapting this technology for use in actual injuries,” said Cohen.
Story Source:
The above story is based on materials provided by UC Berkeley NewsCenter, Sarah Yang.