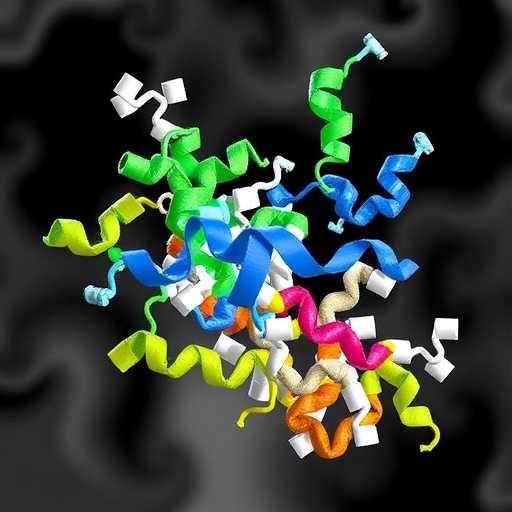
In a landmark study revealing new dimensions of enzyme regulation, researchers have unveiled the intricate molecular mechanism of CSN5i-3, a groundbreaking inhibitor targeting the COP9 signalosome (CSN). Using state-of-the-art cryo-electron microscopy (cryo-EM), the team delineated the precise binding mode of CSN5i-3 within the intact CSN complex, providing unmatched insights into its role as a hybrid orthosteric uncompetitive inhibitor. This discovery not only deepens our understanding of CSN’s enzymatic control but also opens novel avenues for therapeutic interventions targeting protein homeostasis pathways.
The COP9 signalosome, a multi-subunit protein complex, plays a pivotal role in regulating cullin-RING ligases (CRLs)—key E3 ubiquitin ligases responsible for protein degradation. CSN’s enzymatic activity relies on the deneddylation of cullin substrates, a process critical to maintaining cellular proteostasis. Central to this function is the catalytic subunit CSN5, whose active site was probed to reveal how inhibition by CSN5i-3 modifies its function. The new cryo-EM map, resolved at an impressive 3.3 Å resolution, captures CSN5i-3 nestled precisely within the catalytic cleft of CSN5, mimicking substrate engagement while imposing steric hindrance.
Intriguingly, the inhibitor displaces a regulatory structural element known as the Ins-1 loop, a key auto-inhibitory segment of CSN5. This displacement occurs independent of neddylated cullin-RING substrates, highlighting CSN5i-3’s ability to access the active site directly, effectively “unlocking” the enzyme in its apo form. This unique mechanism contrasts with canonical inhibitors that typically compete with the substrate binding site without inducing conformational changes. The structural superimposition with the CSN^DM–N8~CLR1 complex demonstrates how CSN5i-3’s binding sterically conflicts with the neddylated cullin’s C-terminal tail and its crucial iso-peptide bond, thus confirming an orthosteric mode of inhibition.
Biophysical assays revealed further fascinating features of CSN5i-3. Through biolayer interferometry (BLI), researchers measured a dissociation constant (K_D) for CSN5i-3 binding to a purified CSN5–CSN6 heterodimer at approximately 4 μM. This moderate affinity was confirmed by isothermal titration calorimetry (ITC), debunking prior reports which suggested an exceptionally high potency in the low nanomolar range. The revision in binding affinity underscores the essential dynamics in the enzyme-inhibitor interaction and hints at allosteric modulation rather than simple competitive binding.
Despite this moderate binding affinity, CSN5i-3 showed remarkable potency in biochemical assays, achieving inhibition of CSN’s isopeptidase activity toward N8-conjugated CRL1 (N8~CRL1) substrates with an IC_50 of just 29 nM. This discrepancy implies that CSN5i-3 preferentially binds to the enzyme-substrate complex rather than the isolated enzyme, marking it as an uncompetitive inhibitor. This mode of action is rare and positions CSN5i-3 as a hybrid molecule that acts orthosterically within a substrate-induced conformational landscape.
Mechanistically, the substrate-induced remodeling of the Ins-1 loop appears to create a more favorable binding pocket for CSN5i-3 by transiently exposing the active site. This substrate-driven conformational plasticity facilitates the inhibitor’s access, effectively “gluing” itself within the active site and preventing catalysis. This dual dependency on substrate presence for high-affinity binding renders CSN5i-3 a molecular glue, stabilizing an inhibited conformation of the CSN complex. Such molecular glues have recently emerged as promising drug modalities due to their specificity and unique allosteric effects.
The structural and functional characterization of CSN5i-3 stands to influence drug discovery paradigms targeting the ubiquitin-proteasome system and protein degradation machinery. By exploiting conformational dynamics intrinsic to CSN activation, CSN5i-3 exemplifies a sophisticated approach to enzyme inhibition that can discriminate between enzyme states, reducing off-target effects and improving pharmacological profiles. This layered mechanism of action suggests future designs of allosteric or substrate-dependent inhibitors across diverse enzymatic families.
Moreover, this study leverages cryo-EM techniques that capture not only static snapshots but also dynamic landscapes of multimeric protein complexes, underlining the profound impact of high-resolution structural biology in revealing inhibitor modes. The work illustrates how combining structural elucidation with biophysical and biochemical assays provides a comprehensive framework to understand enzyme regulation and inhibition deeply.
The implications for cancer biology and other disease areas are significant. Dysregulation of the COP9 signalosome and its associated CRL pathways contributes to many malignancies, making CSN5 a compelling therapeutic target. Molecules like CSN5i-3, with their precise mode of action, could be tailored to modulate protein degradation in cells selectively, paving the way for novel anti-cancer agents with enhanced specificity and reduced toxicity.
Concluding, these findings mark a milestone in understanding the molecular basis of CSN inhibition. CSN5i-3’s characterization as an orthosteric molecular glue inhibitor not only challenges traditional views of enzyme inhibition but also enriches the chemical toolbox for manipulating ubiquitin signaling pathways. The extensive integrative approach embodied by this research holds promise for future breakthroughs in targeted therapeutics and molecular pharmacology.
As the therapeutic landscape increasingly embraces complex allosteric and substrate-dependent mechanisms, molecules like CSN5i-3 will likely spearhead innovation, offering unprecedented control over key cellular machines. Their discovery underscores the transformative power of modern structural biology and chemical biology in decoding and controlling fundamental biological processes at the molecular level, offering hope for next-generation interventions.
With this detailed portrait of CSN5i-3, the frontier of enzyme inhibition is redefined, showcasing how molecular glues can elegantly exploit enzyme conformations and substrate-induced activation states to achieve selective, potent inhibition.
Subject of Research: Mechanistic characterization of CSN5i-3 inhibition of the COP9 signalosome.
Article Title: CSN5i-3 is an orthosteric molecular glue inhibitor of COP9 signalosome.
Article References: Shi, H., Wang, X., Yu, C. et al. CSN5i-3 is an orthosteric molecular glue inhibitor of COP9 signalosome. Nature (2026). https://doi.org/10.1038/s41586-026-10129-y
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41586-026-10129-y
Tags: COP9 signalosome enzyme regulationcryo-electron microscopy CSN structureCSN5 catalytic subunit inhibitionCSN5i-3 molecular glue inhibitorcullin-RING ligase deneddylationenzyme-substrate mimicry inhibitorshigh-resolution cryo-EM enzyme mappingIns-1 loop displacement CSN5novel E3 ligase regulatoryorthosteric uncompetitive inhibition mechanismprotein homeostasis therapeutic targetsubiquitin-proteasome system regulation