In a groundbreaking advancement that holds transformative potential for the treatment of chronic inflammatory diseases, scientists have engineered exosome nanovesicles designed to deliver therapeutic antibodies directly to sites of inflammation in the gastrointestinal tract. This innovative approach, detailed in the upcoming publication in Nature Communications by Cao, Luo, Miao, and colleagues, represents a significant leap forward in nanomedicine and targeted drug delivery systems for inflammatory bowel disease (IBD), a debilitating condition that affects millions worldwide.
Inflammatory bowel disease, encompassing Crohn’s disease and ulcerative colitis, has long posed immense challenges to clinicians due to its chronic, relapsing nature and the difficulty in precisely targeting inflamed tissues without systemic side effects. Traditional antibody therapies, although effective in certain cases, often suffer from poor bioavailability, rapid clearance from the bloodstream, and off-target effects that can compromise patient safety. Addressing these limitations, the new strategy employs engineered exosome nanovesicles—tiny, lipid-bilayer vesicles naturally secreted by cells and capable of crossing biological barriers—to ferry antibodies with unprecedented precision.
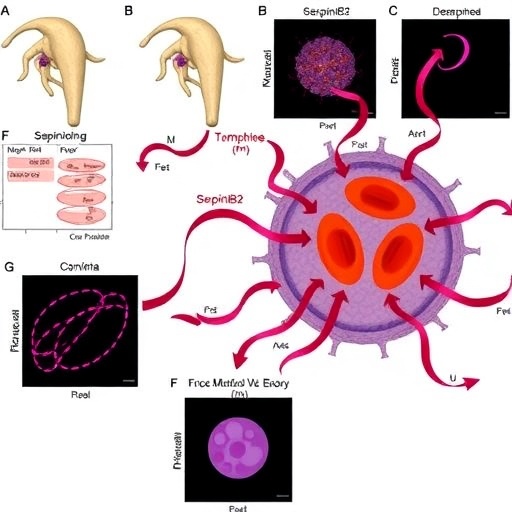
The cornerstone of this technology lies in the bioengineering of exosomes derived from immune cells, tailored to encapsulate monoclonal antibodies against key inflammatory mediators implicated in IBD pathogenesis. These nanovesicles exhibit exceptional stability in the hostile environment of the gastrointestinal tract, enabling the antibodies to survive enzymatic degradation and reach the inflamed mucosa intact. Upon arrival, the exosomes engage with target cells through receptor-mediated mechanisms, facilitating the intracellular delivery of antibodies to modulate aberrant immune responses driving disease progression.
Crucially, the researchers employed cutting-edge molecular techniques to functionalize the exosome surfaces with ligands that selectively bind to adhesion molecules overexpressed in the inflamed intestinal endothelium. This active targeting mechanism enhances the accumulation of therapeutic antibodies exactly where they are needed, minimizing off-target delivery and systemic immunosuppression. The resultant pharmacokinetic profile showed prolonged retention of the antibody payload in diseased tissues, translating to improved efficacy in preclinical IBD models.
In rigorous in vivo experiments involving murine models of colitis, treatment with these engineered exosome nanovesicles led to notable reductions in inflammatory cytokine levels, diminished mucosal ulceration, and restoration of intestinal barrier integrity. These outcomes underscore the potential not only to ameliorate symptoms but also to address the underlying pathophysiological mechanisms at a molecular level. Moreover, the biocompatibility and minimal immunogenicity of the exosome platform bode well for translational applications in human patients.
The integration of nanotechnology with immunotherapy exemplified by this work addresses several bottlenecks that have hindered therapeutic progress in IBD. By leveraging the natural communication pathways of exosomes, the delivery system can bypass biological barriers such as the mucus layer and extracellular matrix, which conventionally hinder antibody penetration into gut tissues. Additionally, this approach mitigates systemic exposure, thereby reducing the risk of adverse effects commonly associated with conventional monoclonal antibody therapies.
Further mechanistic studies uncovered that the delivery of antibodies via engineered exosomes not only neutralizes pro-inflammatory cytokines but also reprograms local immune cell populations. This reprogramming shifts macrophage polarization from a pro-inflammatory M1 phenotype to a regulatory M2 phenotype, fostering an environment conducive to tissue repair and immune homeostasis. Such immunomodulatory effects herald a paradigm shift in the treatment strategies of chronic inflammatory diseases beyond IBD.
The versatility of this platform also opens avenues for its application beyond antibody delivery. By customizing the cargo payload, researchers envision the potential encapsulation of nucleic acids such as siRNAs or therapeutic proteins, enabling combinatorial therapies in a single nanovesicle formulation. This modular design affirms the promise of exosome-based nanocarriers as a multifunctional vehicle in precision medicine.
Notably, the scalability of exosome production was addressed through the development of bioreactor systems optimized for mass culture of donor cells. This advancement ensures adherence to good manufacturing practices (GMP), a critical step toward clinical translation. Coupled with standardized purification protocols and thorough characterization by nanoparticle tracking analysis, electron microscopy, and flow cytometry, the study lays a comprehensive foundation for regulatory approval pathways.
Despite the remarkable progress, challenges remain, such as refining targeting specificity to avoid unintended interactions and ensuring the stability of loaded antibodies during storage and transport. Future studies focusing on humanized models and eventual clinical trials will be critical to affirm therapeutic benefits and safety profiles in diverse patient populations. Importantly, patient stratification based on biomarker profiles may optimize responses to exosome-based antibody therapies.
This pioneering work epitomizes the intersection of bioengineering, immunology, and nanomedicine, offering a beacon of hope for patients grappling with IBD and potentially other inflammatory disorders. As the global burden of chronic inflammatory diseases continues to rise, innovations like engineered exosome nanovesicles herald a new era of targeted, efficient, and safer treatment modalities. The promise of harnessing the body’s own cellular messaging systems to deliver therapeutic payloads with surgical precision not only revolutionizes drug delivery paradigms but also paves the way for personalized medicine tailored to individual disease signatures.
Looking ahead, the collaboration between multidisciplinary research teams, clinicians, and biotech industry stakeholders will be pivotal in accelerating the bench-to-bedside trajectory of this technology. As we edge closer to clinical realization, the prospect of alleviating millions of lives strained by relentless inflammation becomes increasingly tangible. The 2026 publication in Nature Communications will undoubtedly be a milestone reference for future explorations aimed at conquering inflammatory bowel disease through nanotherapeutics.
In conclusion, the engineering of exosome nanovesicles for antibody delivery represents a bold scientific stride with profound therapeutic implications. By surmounting traditional hurdles of antibody therapies and exploiting the inherent biological advantages of exosomes, this novel approach offers a sophisticated, targeted, and potentially transformative treatment for inflammatory bowel disease. The continued pursuit of innovation in this domain promises to unlock new frontiers in the management of not only IBD but a broad spectrum of immune-mediated diseases.
Subject of Research: Engineered exosome nanovesicles for targeted delivery of antibodies in inflammatory bowel disease therapy
Article Title: Engineered exosome nanovesicles for delivery of antibodies to treat inflammatory bowel disease
Article References:
Cao, J., Luo, R., Miao, R. et al. Engineered exosome nanovesicles for delivery of antibodies to treat inflammatory bowel disease. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69382-4
Image Credits: AI Generated