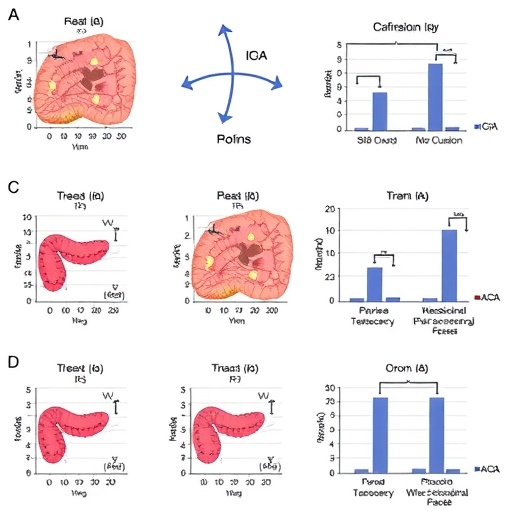
In a groundbreaking study published in Nature Communications, a team of researchers led by Raschdorf, de Almeida, and Solbach has unveiled a compelling molecular link between mitochondrial dysfunction and a deficiency in dimeric IgA-secreting plasma cells, providing novel insights into the complex pathogenesis of Crohn’s disease. Employing state-of-the-art colonic spatial single-cell proteomics alongside rigorous murine models, this investigation dives deep into the cellular intricacies that underpin chronic intestinal inflammation—opening promising avenues for targeted therapeutic strategies.
Crohn’s disease has long been recognized as a multifactorial ailment, with genetic, environmental, and immunological components intertwining to produce debilitating gastrointestinal symptoms. However, this new research highlights mitochondrial impairment within the colonic environment as a critical factor influencing immune dysregulation. The study meticulously maps the spatial proteomic landscape of human colonic tissue at the single-cell level, revealing distinct protein expression patterns that correlate mitochondrial anomalies with a specific deficit in dimeric immunoglobulin A (IgA)-producing plasma cells.
Mitochondria, well known as cellular powerhouses, are fundamentally responsible for energy metabolism and cellular homeostasis. Their malfunction has been implicated in diverse diseases but linking mitochondrial health directly to immunoglobulin secretion within the gastrointestinal tract marks a novel paradigm shift. The researchers utilized advanced imaging mass cytometry techniques combined with single-cell proteomic profiling to spatially resolve mitochondrial enzyme distribution and immune cell phenotypes within Crohn’s disease-affected colonic regions compared to healthy controls.
A critical finding of this investigation is that the compromised mitochondrial function detrimentally affects the production of dimeric IgA, the form predominantly secreted at mucosal surfaces and vital for maintaining intestinal barrier integrity. The depletion of these IgA-secreting plasma cells compromises mucosal immunity, exacerbating susceptibility to microbial dysbiosis—a known driver of chronic inflammation in Crohn’s pathology. By meticulously analyzing these cellular interactions, the authors elucidate how energy deficits at the mitochondria may cascade into impaired immune defenses within the intestine.
To substantiate their human tissue findings, the team deployed genetically engineered murine models exhibiting controlled mitochondrial dysfunction specifically in plasma cells. These mice recapitulated key features observed in patients, including fewer dimeric IgA-secreting plasma cells and increased inflammatory responses in the colon. This cross-species validation highlights the causal relationship between mitochondrial impairment and immune cell depletion, firmly anchoring the hypothesis in experimental evidence.
Furthermore, the spatial resolution afforded by the single-cell proteomics approach uncovered microenvironmental heterogeneity within the colonic mucosa. Pockets of mitochondrial distress were closely associated with regions of inflammatory infiltration and altered immune cell composition. Such fine-grained analysis supports a model wherein mitochondrial health orchestrates localized immune responses, pinpointing potential cellular targets for intervention designed to restore homeostasis.
The study also emphasizes the dynamic role of IgA in preserving the delicate balance between commensal gut microbiota and immune surveillance. The insufficiency of dimeric IgA resulting from mitochondrial deficits disrupts this equilibrium, fostering conditions conducive to pathological inflammation and tissue damage typical of Crohn’s disease. This mechanistic insight underscores the importance of mitochondrial maintenance not only for cellular metabolism but also as a pivotal factor in mucosal immunity.
Intriguingly, the research team explored therapeutic implications by examining whether interventions aimed at enhancing mitochondrial function could rescue plasma cell IgA production. Preliminary murine trials using mitochondrial-targeted antioxidants demonstrated partial restoration of dimeric IgA secretion and reduced inflammatory markers. Although early, these findings pave the way for mitochondrial modulation as a novel therapeutic axis in inflammatory bowel diseases.
Technically, the deployment of cutting-edge single-cell proteomic platforms marks a significant leap forward for immunology research. Integrating spatial context with protein expression at single-cell resolution enables researchers to unravel complex cellular ecosystems within diseased tissues, which traditional bulk analyses obscure. This multidimensional approach yields unprecedented clarity into how subcellular organelle dysfunction translates into pathophysiological outcomes.
Moreover, the sophisticated use of murine genetic models tailored to mimic human mitochondrial impairments provides compelling cause-and-effect relationships rare in human studies. This combination of human tissue analysis with mechanistic murine modeling offers a powerful blueprint for future explorations of immune-metabolic crosstalk in chronic inflammatory disorders.
The implications of this study extend beyond Crohn’s disease, suggesting that mitochondrial health within immune cells could be a generalized determinant of mucosal immunity. Such findings prompt reconsideration of therapeutic strategies targeting metabolic pathways to modulate immune function, potentially impacting a wide spectrum of autoimmune and inflammatory diseases.
This research also highlights the importance of dimeric IgA, often overshadowed by monomeric immunoglobulin isotypes, in sustaining mucosal immunological defenses. By delineating the cellular pathways leading to its deficiency, the study raises awareness of plasma cell heterogeneity and its contribution to gut homeostasis, inviting deeper inquiries into plasma cell biology within mucosal tissues.
While the study promises exciting therapeutic prospects, challenges remain in translating these findings into clinical interventions that can precisely target mitochondrial function within specific immune subsets without unintended systemic effects. The nuanced interplay between metabolism and immunity demands carefully engineered strategies to balance efficacy and safety.
In conclusion, Raschdorf and colleagues have propelled our understanding of Crohn’s disease forward by illuminating how mitochondrial dysfunction undermines a specialized arm of mucosal immunity through dimeric IgA-secreting plasma cell deficiency. Their work elegantly integrates technological innovation with biological insight, laying foundational knowledge that may revolutionize treatment paradigms for patients suffering from this burdensome chronic condition.
Future research building upon these findings will likely explore the therapeutic potential of mitochondrial enhancement and examine other immune cell populations affected by metabolic dysregulation. The convergence of spatial proteomics, genetic modeling, and immunometabolism exemplified in this study heralds a new era for unraveling the cellular underpinnings of complex autoimmune diseases.
As Crohn’s disease affects millions worldwide, elucidating precise molecular mechanisms is critical for advancing patient care. This study represents a vital step toward that goal, inspiring hope for novel interventions that restore intestinal immune equilibrium by targeting mitochondrial vitality at the cellular level.
Subject of Research: The link between mitochondrial dysfunction and deficiency of dimeric IgA-secreting plasma cells in Crohn’s disease.
Article Title: Colonic spatial single-cell proteomics and murine models link mitochondrial dysfunction to dimeric IgA-secreting plasma cell deficiency in Crohn’s disease.
Article References:
Raschdorf, A., de Almeida, L.N., Solbach, P. et al. Colonic spatial single-cell proteomics and murine models link mitochondrial dysfunction to dimeric IgA-secreting plasma cell deficiency in Crohn’s disease. Nat Commun 17, 1590 (2026). https://doi.org/10.1038/s41467-026-69069-w
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41467-026-69069-w
Tags: advanced imaging techniques in medical researchcellular energy metabolism and immunitychronic intestinal inflammation mechanismsdimeric IgA-secreting plasma cellsIgA deficiency and immune dysregulationMitochondrial dysfunction in Crohn’s diseasemitochondrial impairment and immune responsemultidisciplinary approaches in disease studynovel insights into Crohn’s pathogenesisproteomic analysis of gastrointestinal healthsingle-cell proteomics in colonic tissuetargeted therapies for Crohn’s disease