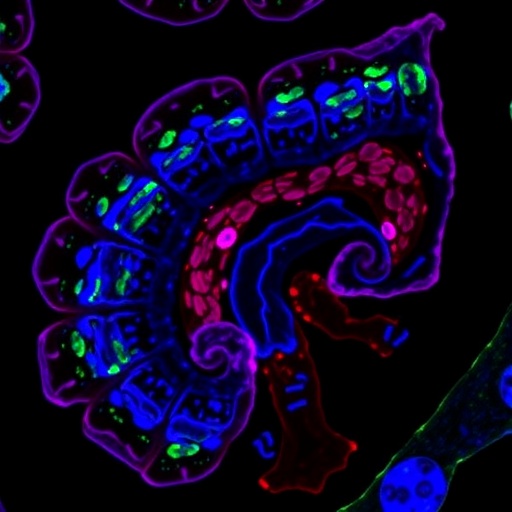
The intestinal epithelium is a remarkable tissue, constantly undergoing renewal every three to five days. This rapid regeneration is fueled by a population of intestinal stem cells (ISCs) residing at the base of the crypts. These ISCs are indispensable for maintaining epithelial integrity and orchestrating tissue repair, particularly after damage induced by insults such as chemotherapy or radiation. However, despite their critical role, the precise molecular mechanisms governing ISC proliferation and differentiation have remained elusive.
Recent groundbreaking research led by Professor Yingying Le at the Shanghai Institute of Nutrition and Health within the Chinese Academy of Sciences has unveiled a crucial regulator in this regenerative process: formyl peptide receptor 2 (FPR2). FPR2 is a G-protein-coupled receptor (GPCR) known primarily for its function as a pattern recognition receptor (PRR). It detects a diverse array of ligands, ranging from bacterial-derived formyl peptides such as N-formylmethionyl-leucyl-phenylalanine (fMLF) to endogenous lipids and synthetic agonists. Traditionally, FPR2 has been studied extensively in phagocytic leukocytes, where it modulates immune responses, inflammation, and wound healing.
What makes this study particularly noteworthy is its focus on FPR2 expression within intestinal epithelial cells (IECs) themselves—a context that had not been explored comprehensively before. Through elegant genetic and cellular approaches, the researchers elucidated how epithelial FPR2 acts as a central node in gut homeostasis and tissue repair, fundamentally altering our understanding of intestinal biology.
Utilizing sophisticated conditional knockout mouse models—including intestinal epithelial-specific Fpr2 knockout (Fpr2^VKO) and ISC-specific knockout models (Lgr5-Fpr2KO)—the research team observed dramatic structural and functional changes in the intestines of Fpr2-deficient animals. These mice exhibited reduced villus height and crypt depth, indicative of compromised epithelial architecture. Furthermore, the populations of goblet cells, Paneth cells, and resident ISCs were significantly diminished. Correspondingly, markers of proliferation such as Ki67 and BrdU incorporation were substantially suppressed, suggesting that both ISCs and transit-amplifying (TA) cells suffered from impaired proliferative capacity.
Delving deeper into cellular signaling networks, the researchers identified that activation of FPR2 initiates a cascade involving the PKC-ERK signaling axis. This kinase pathway subsequently drives the upregulation of hallmark genes associated with the Wnt, Notch, and Hippo pathways—three essential signaling routes for cell cycle progression, differentiation, and organ size regulation. Single-cell RNA sequencing of intestinal organoids confirmed that FPR2 stimulation significantly enhances expression of critical regulators including Wnt3, and Notch pathway components, orchestrating a finely-tuned balance between ISC self-renewal and differentiation.
Intriguingly, the study also highlights a potential interplay between the gut microbiota and epithelial regeneration. The bacterial chemotactic peptide fMLF was found capable of mimicking the effects of FPR2 activation in an FPR2-dependent manner. This suggests that microbial-derived signals could participate in modulating gut epithelial homeostasis through FPR2 engagement, opening new avenues for microbiota-targeted therapeutic strategies.
Functional validation came from injury models simulating clinical scenarios of intestinal damage. Both X-ray irradiation and 5-fluorouracil (5-FU) treatment inflicted severe epithelial injury, and in such contexts, Fpr2-knockout mice demonstrated delayed epithelial regeneration and poorer survival outcomes. Remarkably, administration of MMK-1, a synthetic FPR2 agonist, counteracted these deficits by accelerating tissue repair processes and enhancing survival rates. These compelling findings underscore the therapeutic potential of FPR2 agonists as adjuncts in mitigating intestinal toxicity—a major limitation in cancer treatments.
This study’s revelation that intestinal epithelial FPR2 is a master regulator linking innate immune sensing to cellular proliferation and differentiation paradigms revolutionizes our perspective on gut biology. Crucially, it presents a unifying molecular axis through which environmental and microbial cues can directly influence stem cell-driven epithelial renewal and repair mechanisms. This insight holds immense promise for the development of novel interventions aimed at treating intestinal injury and inflammatory bowel conditions.
Future investigations are expected to focus on identifying endogenous ligands within the gut milieu that naturally modulate FPR2 signaling under physiological and pathological conditions. Additionally, there is a tremendous interest in advancing this knowledge toward clinical application by designing FPR2-targeted therapeutics tailored to enhance epithelial regeneration without triggering deleterious inflammatory responses.
In summary, Professor Yingying Le’s team has elegantly demonstrated that epithelial FPR2 functions at the frontier of host-microbe crosstalk, orchestrating intestinal stem cell fate decisions through the PKC-ERK-Wnt/Notch/Hippo signaling network. These insights not only enrich the fundamental understanding of intestinal physiology but also lay the groundwork for groundbreaking therapeutics poised to improve outcomes for patients suffering from chemotherapy- or radiation-induced gut injury and beyond.
Subject of Research: Experimental study on intestinal stem cell regulation and epithelial homeostasis
Article Title: Intestinal epithelial formyl peptide receptor 2 contributes to intestinal epithelium homeostasis and injury repair by regulating intestinal stem cell and transit-amplifying cell proliferation and differentiation
News Publication Date: 24-Dec-2025
Web References: http://dx.doi.org/10.1093/lifemeta/loaf045
Image Credits: HIGHER EDUCATION PRESS
Keywords: Cell biology, intestinal stem cells, epithelial regeneration, formyl peptide receptor 2, PKC-ERK signaling, Wnt pathway, Notch signaling, Hippo pathway, gut microbiota, tissue repair, chemotherapy-induced injury
Tags: cellular mechanisms of intestinal repairchemotherapy effects on intestinal tissueformyl peptide receptor 2 functions in epithelial cellsFPR2 role in intestinal epithelial repairG-protein-coupled receptors in tissue regenerationimmune response modulation by FPR2intestinal epithelium regeneration after injuryintestinal stem cells proliferation mechanismspattern recognition receptors in gut healthradiation damage recovery in intestinal epitheliumresearch on intestinal epithelial cell signalingShanghai Institute of Nutrition and Health research findings