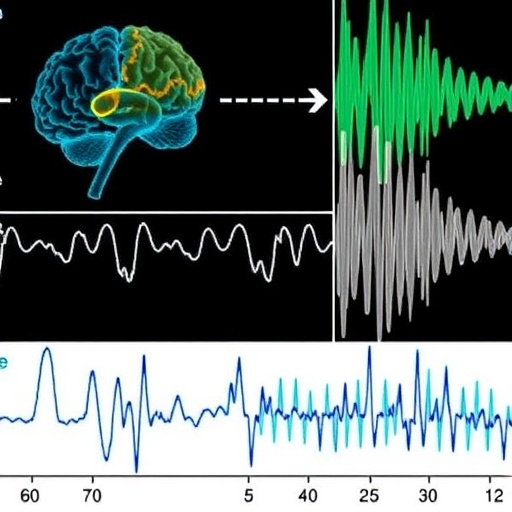
In a groundbreaking study published in Nature Communications, researchers at the Massachusetts Institute of Technology (MIT) have unveiled a novel electrophysiological biomarker of Fragile X syndrome—offering unprecedented translational promise for modeling and treating this most common inherited form of autism. This discovery bridges a crucial gap that has long hindered effective drug development for neurodevelopmental disorders: the absence of objective and non-invasive measures consistently shared between human patients and animal models.
Led by postdoctoral researcher Sara Kornfeld-Sylla and marked by the leadership of Picower Professor Mark Bear, the team conducted parallel EEG recordings in fragile X-affected males and genetically analogous Fmr1-/y mice. Their innovative signal processing approach isolated subtle yet robust alterations in low-frequency brain oscillations, revealing conserved electrophysiological “signatures” relevant across species and developmental stages. This cross-species coherence in brainwave dynamics paves the way for a reliable translational biomarker that could revolutionize preclinical assessment and accelerate therapeutic pipelines.
The study’s crux rested on examining occipital lobe brainwaves—specifically from the visual cortex—in both human subjects and mice. The MIT group deliberately moved beyond conventional EEG frequency band categorizations (delta, theta, alpha, beta, gamma) to more precisely compare periodic spectral power devoid of background noise. This technique uncovered a striking shift: Fragile X individuals and mouse models exhibited markedly slower peak frequencies within low-frequency spectra, yet the specific bands affected were not identical. Humans demonstrated shifts in the alpha range, whereas mice showed similar phenomena in the theta band, underscoring the importance of evaluating spectral power in a granular, band-agnostic manner rather than matching classical frequency designations rigidly.
Intriguingly, these altered neurophysiological patterns evolved with age. In adult males and mature mice, Fragile X syndrome was characterized by a prominent slowing of the peak low-frequency oscillations, whereas in youthful boys and juvenile mice, power reduction at these peaks was more salient, highlighting a developmental trajectory of electrophysiological dysfunction. Such nuanced differentiation in brainwave features across ages adds a compelling dimension to understanding the neurobiology of Fragile X and possibly other neurodevelopmental disorders.
Delving deeper into the microscopic origins of these EEG biomarkers, the team implanted fine probes into the visual cortex of awake mice to dissect the composite subpeaks underlying these low-frequency oscillations. They determined that the key fragile X-related biomarker corresponds to the more slowly oscillating subpeak within the broader spectrum. This meticulous ‘under the hood’ investigation linked the marker to specific inhibitory neural circuits, particularly somatostatin-expressing interneurons, a specialized class of neurons known to sculpt rhythmic brain activity by modulating excitatory cell firing through GABAergic inhibition.
To causally validate the connection between interneuronal activity and the Fragile X biomarker, researchers selectively suppressed either somatostatin or parvalbumin interneurons in mice. The somatostatin neuronal manipulation distinctly perturbed the low-frequency subpeak identified as the electrophysiological signature of Fragile X, thereby clarifying the mechanistic foundation of the biomarker. These data implicate disrupted inhibitory control as a key pathophysiological process underpinning Fragile X’s neurofunctional deficits.
Therapeutically, the research team investigated the modulation of this biomarker by arbaclofen—a GABA-B receptor agonist known to potentiate inhibitory neurotransmission—previously considered a promising candidate treatment for Fragile X. Administering arbaclofen to both wild-type and Fragile X model mice revealed dose-dependent amelioration of the electrophysiological abnormalities: even low acute doses enhanced the biomarker’s power in control animals, while higher doses normalized the reduced peak power in fragile X juveniles. This provides compelling proof-of-concept that the biomarker not only reflects disease pathology but can serve as an objective readout for drug efficacy.
This cross-species electrophysiological marker offers a unique translational bridge previously lacking in fragile X and autism research. By enabling dose mapping between preclinical models and human patients, it empowers more precise evaluation of pharmacodynamic effects on neural circuit function, reducing the attrition rate of candidate drugs in clinical trials. Moreover, through its sensitivity to acute drug modulation, it opens avenues for rapid screening of novel therapeutic agents aimed at restoring cortical inhibition deficits.
Beyond Fragile X, Kornfeld-Sylla and colleagues suggest this biomarker framework might extend to other brain disorders featuring alpha rhythm abnormalities such as schizophrenia and epilepsy. Identification of broadly conserved electrophysiological phenotypes could transform translational neuroscience research, fostering rapid biomarker-guided drug discovery across a systematic spectrum of neurodevelopmental and neuropsychiatric diseases.
The collective effort involved key collaborations spanning Boston Children’s Hospital, the Phelan-McDermid Syndrome Foundation, Cincinnati Children’s Hospital, the University of Oklahoma, and King’s College London—facilitating data aggregation and cross-validation. These partnerships underscore the contemporary imperative for data sharing in tackling complex brain disorders.
In summary, this landmark research elucidates how low-frequency brainwave abnormalities in the primary visual cortex constitute a shared electrophysiological marker of Fragile X syndrome in humans and mice. The rigorous mechanistic and pharmacological dissection of this biomarker heralds a new era for objective, cross-species biomarkers in neuroscience, poised to underpin future therapeutic breakthroughs in autism spectrum disorders.
Subject of Research: People
Article Title: A human electrophysiological signature of Fragile X pathophysiology is shared in V1 of Fmr1-/y mice
News Publication Date: 9-Feb-2026
Web References: https://dx.doi.org/10.1038/s41467-026-69243-0
Image Credits: Bear Lab/MIT Picower Institute
Keywords: Autism, Fragile X syndrome, Neuroscience, Biomarkers, Neurology, Brain, Translational medicine
Tags: brainwave dynamics comparisondrug development for autismEEG recordings in autismelectrophysiological signaturesFragile X syndrome biomarkerhuman and mouse modelslow-frequency brain oscillationsMIT research on autismNeurodevelopmental Disorderspreclinical assessment techniquestranslational research in Fragile Xvisual cortex brainwaves