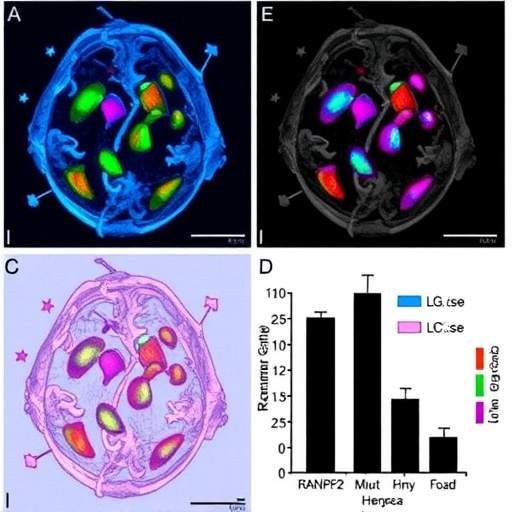
In a groundbreaking study published in Nature Communications in 2026, researchers have unveiled critical insights into the molecular mechanisms driving Acute Necrotizing Encephalopathy (ANE), a devastating neurological condition often triggered by viral infections such as Influenza A. The team, led by Desgraupes, Decorsière, Perrin, and colleagues, has identified the genetic regulator RANBP2 as a pivotal factor that modulates the inflammatory response to Influenza A virus infection, shedding light on potential therapeutic targets for this rare but deadly disease.
Acute Necrotizing Encephalopathy is characterized by rapid and severe brain inflammation predominantly affecting children and young adults. The condition follows viral infections, and its sudden onset leads to widespread neuronal damage, resulting in a poor prognosis and high mortality rates. Despite its severity, the molecular drivers of ANE have remained elusive, complicating efforts to develop effective treatments. This newly published research delineates the role of the RANBP2 gene as a key orchestrator in the immune system’s response to viral assaults.
RANBP2, an essential nucleoporin, is traditionally known for its involvement in nuclear-cytoplasmic transport. However, this study reveals that RANBP2 extends far beyond structural cellular functions. It acts as a genetic switch that influences the body’s inflammatory cascade following Influenza A virus infection. The researchers demonstrated through extensive in vitro and in vivo models that mutations or dysregulation in RANBP2 amplify inflammatory signaling pathways, precipitating the neuropathological features characteristic of ANE.
The team employed sophisticated gene-editing techniques to create cell and animal models deficient in functional RANBP2. These models exhibited exaggerated inflammatory responses when exposed to Influenza A virus, highlighting the gene’s role in tempering immune activation. Notably, the study found that RANBP2 interacts with critical immune modulators, including type I interferons and nuclear factor kappa B (NF-κB), which are central to antiviral defense and inflammation.
One of the most compelling findings is the elucidation of RANBP2’s influence on the production of pro-inflammatory cytokines. In the absence of proper RANBP2 function, the researchers observed a cytokine storm-like phenomenon, where excessive immune signaling leads to neuronal damage and blood-brain barrier disruption. This mechanism is believed to underlie the rapid progression and severity of ANE following viral infections such as flu.
The study further explores how RANBP2 mutations affect the central nervous system’s innate immunity. Normally, RANBP2 helps maintain a delicate balance between protective antiviral responses and preventing excessive inflammation. The loss of this balance results in unchecked inflammatory pathways, promoting glial activation and neuronal death—hallmarks of ANE pathology. Such insights deepen our understanding of how genetic predispositions can modulate disease outcomes in viral encephalopathies.
Additionally, these findings have broad implications beyond ANE and Influenza A infections. Since RANBP2 is ubiquitously expressed and involved in fundamental cellular processes, its role in immune regulation might extend to other viral infections and inflammatory neurological disorders. This opens exciting avenues for research into common molecular underpinnings of virus-induced neuroinflammation.
The researchers also investigated therapeutic strategies to mitigate the adverse effects of RANBP2 dysregulation. By employing pharmacological inhibitors that target downstream inflammatory mediators such as NF-κB and cytokine production pathways, they successfully reduced neuroinflammation and improved survival rates in animal models. These promising results suggest that modulating RANBP2-related pathways could become a viable approach for treating ANE and potentially other virus-associated neuroinflammatory conditions.
Importantly, the research highlights the necessity of early genetic screening in patients with severe viral encephalopathies to identify RANBP2 mutations. Such diagnostics could enable personalized medicine approaches, allowing clinicians to tailor immunomodulatory therapies timely and effectively. This paradigm shift could significantly enhance patient outcomes by preventing or attenuating the neurological damage characteristic of ANE.
Moreover, the study underscores the dynamic interplay between host genetics and viral pathogens in shaping disease severity. The intricate regulation of inflammation by RANBP2 exemplifies how a single gene’s functional status can dramatically influence the trajectory of infection and inflammation. This realization marks a step forward in the precision medicine era, emphasizing genomic context in infectious disease management.
The research team utilized cutting-edge molecular biology tools, including CRISPR/Cas9 genome editing, transcriptomic profiling, and proteomics, to dissect RANBP2’s multifaceted role. This integrative approach allowed them to map the complex signaling networks influenced by RANBP2 and identify novel interaction partners involved in antiviral immunity and inflammation control.
Findings from this comprehensive investigation also raise critical questions about the evolutionary conservation of RANBP2 functions and its involvement in immune responses across species. Understanding these aspects could inform the development of cross-species models for studying viral encephalitis and refining therapeutic approaches based on evolutionary biology principles.
In the broader context of neurovirology, this study exemplifies the importance of dissecting host-pathogen interactions at the genetic and molecular levels. By pinpointing RANBP2 as a genetic driver of inflammation in response to Influenza A, the research offers a tangible target for drug development—a crucial step toward mitigating the global burden of viral encephalopathies.
As Influenza A remains a persistent global health challenge with periodic outbreaks and pandemics, unraveling genetic factors like RANBP2 that exacerbate disease outcomes is vital. These new insights equip the scientific and medical communities with knowledge that could transform treatment strategies for viral-induced brain inflammation and improve survival and quality of life for affected patients worldwide.
Ultimately, the work by Desgraupes and colleagues is poised to galvanize further research into the genetic determinants of neuroinflammation and their intersection with infectious diseases. By illuminating the pathways regulated by RANBP2, they offer hope for targeted interventions against one of the most severe complications arising from common viral infections.
This landmark discovery stands as a testament to the power of interdisciplinary collaboration combining genetics, virology, neurology, and immunology, fostering a comprehensive understanding of devastating neurological disorders. As the science unfolds, therapeutic breakthroughs inspired by these findings could revolutionize care for patients afflicted by Acute Necrotizing Encephalopathy and related viral encephalitides.
Subject of Research: The genetic regulation of inflammatory responses in Acute Necrotizing Encephalopathy, specifically focusing on the role of the RANBP2 gene in modulating immune reactions to Influenza A virus infection.
Article Title: The genetic driver of Acute Necrotizing Encephalopathy, RANBP2, regulates the inflammatory response to Influenza A virus infection.
Article References:
Desgraupes, S., Decorsière, A., Perrin, S. et al. The genetic driver of Acute Necrotizing Encephalopathy, RANBP2, regulates the inflammatory response to Influenza A virus infection. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69288-1
Image Credits: AI Generated
Tags: Acute Necrotizing Encephalopathy mechanismsbrain inflammation and injuryimmune system modulationinflammatory response regulationinfluenza A virus infectionmolecular drivers of acute encephalopathyneurological conditions in childrennucleoporins in diseaseRANBP2 gene functionresearch on viral encephalopathytherapeutic targets for encephalopathyviral infection complications