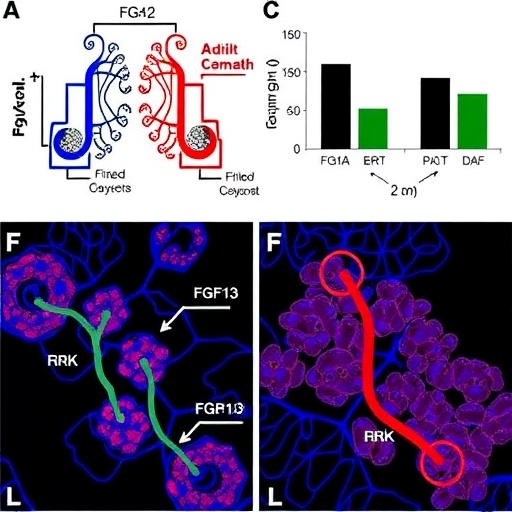
In a groundbreaking advance poised to reshape our understanding of septic lung injury, Zhu, Wang, Jiang, and colleagues have unveiled a pivotal molecular mechanism elucidating how inflammation intensifies metabolic reprogramming within the lungs during sepsis. Published in Nature Communications in 2026, their study spotlights the fibroblast growth factor 13 (FGF13) as a crucial regulator modulating the ERK signaling pathway and aerobic glycolysis in inflammatory lung tissue. This discovery offers profound insights into the molecular interplay underlying sepsis-induced lung damage, presenting promising new targets for therapeutic intervention in a condition notorious for its high mortality and limited treatment options.
Sepsis remains a formidable clinical challenge characterized by systemic inflammation and multi-organ dysfunction, with the lungs often being the first and most severely affected organ. The development of septic lung injury, or acute respiratory distress syndrome (ARDS), involves a complex cascade of molecular events that perturb normal cellular metabolism and immune responses. The study by Zhu et al. provides a detailed mechanistic view of how FGF13 orchestrates these pathological alterations, particularly focusing on the interface between inflammatory signaling and metabolic reprogramming in pulmonary tissues.
At the heart of the investigation is the extracellular signal-regulated kinase (ERK) pathway, a fundamental component of cellular signaling that regulates proliferation, differentiation, and survival. Under septic conditions, ERK activation is heightened, contributing to exacerbated inflammatory responses. Zhu and colleagues demonstrate that FGF13 acts upstream of ERK, serving as a molecular switch that intensifies ERK phosphorylation in alveolar cells. This amplification of ERK signaling is shown to promote a metabolic shift toward aerobic glycolysis, the preferential use of glucose via glycolysis even in the presence of sufficient oxygen—an adaptation often referred to as the Warburg effect in cancer cells but increasingly recognized in inflammatory cells.
Aerobic glycolysis in inflammatory states supports the bioenergetic and biosynthetic demands of activated immune cells, sustaining cytokine production and cellular proliferation. The elucidation of FGF13’s role as a regulator of this metabolic pathway situates it as a master modulator of inflammation during septic lung injury. By fostering ERK-driven glycolytic flux, FGF13 essentially fuels the inflammatory milieu, exacerbating lung tissue injury and dysfunction.
The methodology employed by the researchers incorporated cutting-edge molecular biology techniques, including gene knockout models, metabolic flux analyses, and phosphoproteomic profiling, enabling comprehensive interrogation of FGF13’s regulatory functions. Their in vivo studies, utilizing murine models of sepsis, revealed that deletion or pharmacological inhibition of FGF13 significantly attenuated ERK activation and glycolytic metabolism, resulting in reduced inflammatory cytokine levels and improved pulmonary function. These findings underscore the therapeutic potential of targeting FGF13 to modulate pathogenic inflammation in septic lungs.
Furthermore, the researchers uncovered that FGF13 expression is upregulated in response to inflammatory stimuli such as lipopolysaccharide (LPS), the endotoxin responsible for triggering sepsis in bacterial infections. This upregulation creates a feedback loop whereby heightened FGF13 activity perpetuates ERK signaling and metabolic reprogramming, exacerbating tissue damage. Interrupting this vicious cycle may offer a novel strategy to halt the progression of septic lung injury.
This study also sheds light on the broader implications of metabolic reprogramming in immune regulation. It challenges the traditional view of inflammatory pathways as isolated signaling events, integrating metabolic alterations as central components driving disease pathogenesis. By positioning FGF13 at the nexus of signaling and metabolism, the work opens new frontiers in immunometabolism research, suggesting that modulating metabolic enzymes and regulators could have profound effects on inflammatory diseases beyond sepsis.
Critical to the translational potential of these findings is the identification of small molecules capable of selectively inhibiting FGF13 or disrupting its interaction with the ERK pathway. Early-stage screening reported in the study highlights candidate compounds that reduce FGF13 expression or activity, offering a blueprint for drug development pipelines. Although these molecules require rigorous validation and safety profiling, their discovery is an encouraging step toward targeted therapies for patients suffering from septic lung injury.
The research further elaborates on the pathological consequences of unchecked aerobic glycolysis in alveolar macrophages and epithelial cells. Excess lactate production, a byproduct of enhanced glycolysis, contributes to extracellular acidification, impaired cellular function, and promotes fibrotic remodeling in lung tissue. By attenuating FGF13 activity, the authors propose, it may be possible not only to mitigate acute inflammation but also to prevent long-term complications such as fibrosis and chronic respiratory insufficiency.
Remarkably, the authors employed single-cell RNA sequencing to unravel cell-type specific expression patterns of FGF13 in lung tissues during septic conditions. This approach revealed a heterogeneous landscape where FGF13 was predominantly expressed in pro-inflammatory macrophage subsets and injured epithelial cells, delineating the cellular players most influenced by its regulatory functions. Understanding this spatial and cellular specificity is vital for designing interventions that target pathogenic cells without disrupting homeostatic functions.
The significance of this research extends to the broader context of critical care medicine. Sepsis-induced ARDS remains one of the most lethal complications in intensive care units worldwide, with mortality rates exceeding 40%. Current treatments are largely supportive, including mechanical ventilation and fluid management, underscoring the urgent need for molecularly targeted therapies. By defining a novel regulatory axis controlled by FGF13, this work lays the foundation for a paradigm shift in how clinicians and scientists approach sepsis treatment.
In addition to its mechanistic revelations, the study prompts important questions about the regulation of FGF13 itself. What upstream signals trigger its upregulation? How is its activity fine-tuned in the interplay between immune and structural cells of the lung? Addressing these questions may reveal additional layers of control in the inflammatory response and identify further targets for therapeutic exploitation.
The authors also consider the potential systemic effects of modulating FGF13. Given its involvement in cellular metabolism and signaling pathways shared across tissues, any therapeutic approach must balance efficacy with the risk of off-target effects. The study’s comprehensive in vivo analyses provide initial safety data, but moving toward clinical application will require nuanced understanding of tissue-specific roles and compensatory mechanisms.
Importantly, Zhu and colleagues propose that the principles uncovered in their study may have relevance beyond septic lung injury. The ERK/aerobic glycolysis axis is implicated in numerous inflammatory and autoimmune disorders, suggesting that FGF13 could serve as a universal modulator of pathological metabolic shifts. This broader applicability enhances the impact of their findings and encourages exploration into chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease.
Their work also exemplifies the power of integrating multidisciplinary approaches—from molecular biology and immunology to bioinformatics and metabolomics—in unraveling complex disease processes. Such integrative frameworks are increasingly vital in contemporary biomedical research, where understanding systems-level interactions can catalyze the discovery of novel biomarkers and therapies.
As interest grows in immunometabolism and targeted anti-inflammatory strategies, the discovery of FGF13 as a central regulator of the ERK/glycolysis interface will undoubtedly stimulate further research. It holds promise for the development of innovative treatments that not only alleviate symptoms but also rectify metabolic dysfunctions driving disease progression.
In conclusion, this seminal study by Zhu, Wang, Jiang, and their team illuminates an intricate molecular circuitry controlling inflammation and metabolism in septic lung injury. By establishing FGF13 as a master regulator of the ERK/aerobic glycolysis axis, it offers a transformative perspective on how sepsis damages lung tissue and opens new avenues for therapeutic intervention. As the search for effective sepsis treatments continues, targeting metabolic checkpoints like FGF13 may mark the next frontier in reducing the burden of this deadly disease.
Subject of Research: Regulation of inflammatory signaling and metabolic pathways in septic lung injury, focusing on FGF13’s modulation of the ERK signaling pathway and aerobic glycolysis.
Article Title: FGF 13 functions as a regulator of the ERK/aerobic glycolysis axis in the inflammatory state during septic lung injury.
Article References:
Zhu, J., Wang, J., Jiang, C. et al. FGF 13 functions as a regulator of the ERK/aerobic glycolysis axis in the inflammatory state during septic lung injury. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69014-x
Image Credits: AI Generated
Tags: acute respiratory distress syndrome mechanismsaerobic glycolysis in lung tissueERK signaling pathway in sepsisFGF13 role in septic lung injuryfibroblast growth factor 13 researchinflammation and metabolism in sepsismetabolic reprogramming in lung inflammationmolecular mechanisms of lung injuryNature Communications sepsis studypulmonary tissue damage in sepsissystemic inflammation and organ dysfunctiontherapeutic targets for septic lung injury