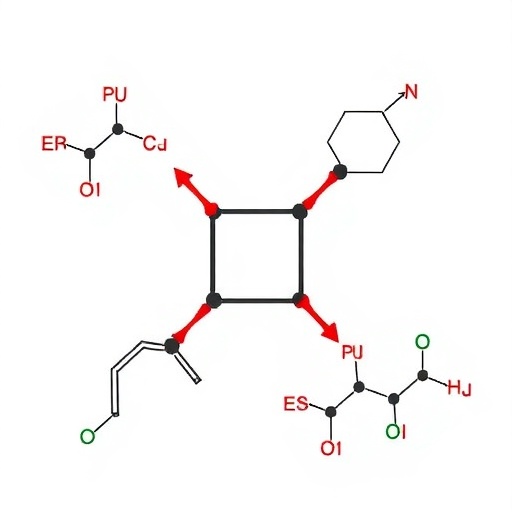
In an exciting advancement for synthetic chemistry, the research group led by Xumu Zhang and Genqiang Chen at the Southern University of Science and Technology has unveiled a groundbreaking method for constructing benzoxane heterocyclic phosphine ligands. This innovation hinges on a tandem nucleophilic addition and aromatic nucleophilic substitution (S_NAr) cyclization process, offering a redox-free, modular synthetic pathway that promises enhanced efficiency and versatility. Their findings have recently been published in the esteemed open-access journal CCS Chemistry, marking a significant leap forward in ligand synthesis techniques.
Benzoxane ligands, recognized for their electron-rich, rigid, and air-stable properties, serve as pivotal components in numerous catalytic processes, especially in transition metal-mediated asymmetric hydrogenations and cross-coupling reactions. Despite their widespread application over the last decade and a half, the synthesis of these ligands has been fraught with challenges largely due to intricate multistep pathways, low selectivity, and cumbersome purification steps. The classical methods typically rely on linear synthetic routes involving redox transformations and the resolution of phosphine oxide intermediates, which hamper scalability and economic feasibility.
Efforts to circumvent these limitations have included condensation reactions between chiral secondary phosphines and ketals. However, such strategies are often plagued by poor diastereoselectivity, necessitating additional resolution or redox steps to achieve optically pure products. Furthermore, existing synthetic routes mostly yield monophosphine or C_2-symmetric bisphosphine frameworks, constraining the structural diversity of obtainable phosphine ligands. Consequently, the demand for an efficient, modular, and broadly applicable methodology to synthesize structurally diverse oxacyclic phosphine ligands has been substantial.
Addressing these longstanding problems, the team ingeniously developed amphiphilic trivalent phosphine reagents, informally dubbed “phosphine click reagents,” to streamline the synthesis of benzoxane ligands. These reagents were synthesized via an elegant two-step, one-pot procedure that demonstrated gram-scale feasibility. Their amphiphilic nature not only afforded enhanced reactivity but also enabled facile manipulation under mild conditions, circumventing the need for harsh redox steps traditionally involved in ligand construction.
Exploiting these phosphine click reagents, the researchers embarked on exploring their reactivity with a variety of aldehydes. Using 1a and benzaldehyde 2a as model substrates in tetrahydrofuran with potassium tert-butoxide as the base, they showcased a remarkable one-step transformation yielding oxoheterocyclic phosphine ligand 3aa in 95% isolated yield and excellent diastereoselectivity (dr > 20:1). The reaction was demonstrated to be amenable to broad substrate scope, including aromatic, heteroaromatic, and even alkyl aldehydes, maintaining high yield and stereoselectivity throughout.
A striking element of this work is the substrate-induced strategy for incorporating chirality. By employing optically pure chiral aldehydes, the team efficiently obtained chiral oxoheterocyclic phosphine ligands without resorting to conventional chiral resolution procedures, thereby simplifying the synthetic route significantly. Furthermore, the methodology was extended to epoxide substrates, facilitating the synthesis of benzoxane-6 oxocyclic phosphine ligands, thereby highlighting the modular and versatile nature of this approach.
To underscore the practical applicability of their chemistry, gram-scale syntheses were conducted, successfully scaling up the process without compromising yield or stereoselectivity. The functional utility of these ligands was demonstrated in asymmetric catalysis: chiral ligand 3ezp exhibited excellent performance in rhodium-catalyzed asymmetric dehydrogenation cross-coupling as well as in palladium-catalyzed asymmetric axial chiral Heck reactions. Equally promising, ligand 3ezs delivered high enantioselectivity (er ~ 96.9:3.1) in iridium-catalyzed asymmetric hydrogenations, emphasizing their value in key synthetic transformations.
Moreover, racemic ligands 3en and 3fn were competent catalysts for palladium-mediated cross-coupling reactions, effectively promoting the formation of carbon-carbon, carbon-nitrogen, and carbon-oxygen bonds. This multifunctionality enhances the appeal of the new ligand class for widespread application in synthetic organic and organometallic chemistry, potentially superseding traditional phosphine ligands in various catalytic domains.
The authors envisaged that the high diastereoselectivity observed primarily stems from the intramolecular S_NAr cyclization step, which involves a significant energy barrier, rather than from the initial nucleophilic addition. This hypothesis was substantiated through meticulous control experiments complemented by insightful theoretical calculations. The presence of a strong base was shown to facilitate generation of nucleophilic phosphine potassium intermediates, which orchestrate the cyclization cascade, reinforcing the mechanistic understanding of this tandem reaction.
This pioneering study outlines a redox-free, modular synthetic platform that not only simplifies the preparation of benzoxane heterocyclic phosphine ligands but also opens avenues for designing structurally diverse and chiral phosphorus-based ligands. By bypassing conventional resolution processes and employing a unique substrate-induced chirality strategy, it offers an innovative framework for future ligand development, with broad implications for asymmetric catalysis and beyond.
Funding and support for this research were generously provided by several prestigious programs, including the National Key Research and Development Program of China, the National Natural Science Foundation of China, Guangdong Provincial Key Research and Development Program, Shenzhen Basic Research Key Project, and the China Postdoctoral Science Foundation, underscoring the strategic importance of this work in advancing chemical sciences.
The publication in CCS Chemistry, a flagship journal of the Chinese Chemical Society dedicated to disseminating groundbreaking chemistry research with diamond open access, ensures broad accessibility and impact. With this novel methodology now accessible, the chemical community can anticipate a surge in ligand innovation aimed at tackling contemporary challenges in catalysis and synthetic design.
The discovery of this robust ambiphilic phosphine reagent and its application to oxyheterocyclic ligand synthesis embodies a significant milestone, promising to reshape the synthetic landscape of phosphorus chemistry. As catalytic processes continue to underpin the creation of valuable chiral molecules, these modular oxacyclic phosphine ligands are poised to become fundamental tools driving the next generation of chemical transformations.
Subject of Research: Not applicable
Article Title: Redox-Free and Modular Access to Oxacyclic Phosphines Enabled by a Robust Ambiphilic Phosphine Reagent
News Publication Date: 8-Dec-2025
Web References:
https://www.chinesechemsoc.org/journal/ccschem
http://dx.doi.org/10.31635/ccschem.025.202506531
Image Credits: CCS Chemistry
Keywords
Ligands
Tags: advancements in synthetic chemistry researchasymmetric hydrogenation improvementsbenzoxane heterocycles applicationschallenges in ligand synthesisdiastereoselectivity in phosphine synthesiselectron-rich phosphine ligandsmodular synthetic pathways in chemistryredox-free ligand synthesis techniquesS_NAr cyclization in synthetic chemistrystreamlined synthesis of phosphine ligandstandem nucleophilic addition methodstransition metal-mediated reactions