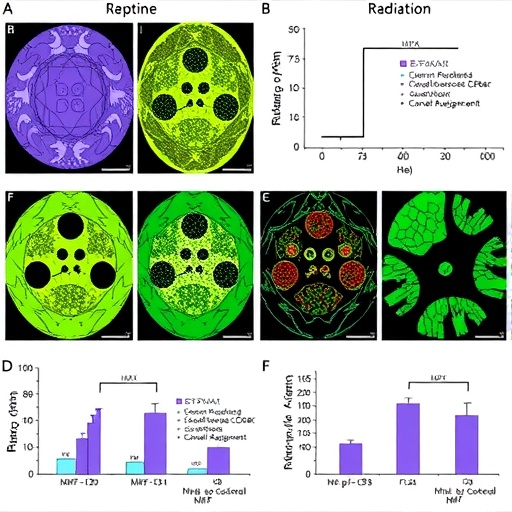
In an innovative study, Zhang and colleagues have unveiled promising findings that highlight a novel approach to alleviating radiation-induced damage in intestinal tissues. Radiation therapy is a cornerstone in cancer treatment; however, its collateral effects often lead to significant morbidity due to damage to the gastrointestinal tract. This research opens avenues for therapeutic interventions by utilizing low doses of 5-hydroxymethylfurfural (5-HMF), a compound derived from natural sources, to counteract such damage.
The implications of radiation-induced intestinal toxicity are profound, as patients undergoing radiation therapy face a myriad of challenges, including inflammation, cell apoptosis, and disruption of the intestinal barrier. These complications not only worsen the quality of life but can also compromise the effectiveness of cancer therapies. Hence, the importance of identifying agents that can bolster intestinal resilience during radiation exposure cannot be overstated.
The study presents compelling evidence suggesting that low-dose 5-HMF administration can substantially enhance the body’s intrinsic ability to cope with radiation-induced stress. The researchers meticulously illustrated how 5-HMF modulates key biological pathways, particularly focusing on the enhancement of the HIF2α-driven IL22/STAT3 signaling axis. This is a critical finding, as the IL-22 cytokine has been widely recognized for its protective role in intestinal health, mediating tissue repair and protective immunity.
Zhang and his team employed sophisticated experimental methodologies, utilizing both in vitro and in vivo models to ascertain the protective effects of 5-HMF on intestine tissues. Their findings consistently indicate that the low-dose application not only reduced intestinal inflammation but also significantly alleviated symptoms associated with radiation exposure, providing a multi-layered defense mechanism against cell stress and apoptosis.
What makes this study exceptionally noteworthy is its exploration of the signaling pathways influenced by 5-HMF. The activation of HIF2α serves not only to stabilize the cellular environment during acute stress but also to trigger a robust inflammatory response that aids in tissue recovery. This dual role underscores the compound’s potential in clinical applications, offering both immediate and prolonged benefits for patients undergoing radiation therapy.
Moreover, the research illustrates the mechanistic details of how IL-22, under the modulation of 5-HMF, upregulates protective genes while simultaneously downregulating pro-inflammatory mediators. This selective targeting is crucial, as it presents a refined method to mitigate adverse effects while enhancing reparative processes, suggesting a pathway toward more effective management strategies for patients suffering from radiation-related side effects.
Interestingly, the study delves into the dose-dependent effects of 5-HMF. While the low dose demonstrated significant protective benefits, higher concentrations may yield diminishing returns or even exacerbate toxicity. This precision in dosing emphasizes the need for careful consideration in clinical settings, ensuring that the therapeutic effect maximizes patient welfare without introducing new risks.
As the field of cancer therapy continuously evolves, integrating compounds such as 5-HMF into treatment regimens could revolutionize the way clinicians approach patient care. The potential for 5-HMF to become a standard adjunct therapy during radiation treatment could optimize patient outcomes significantly, paving the way for further research into its applications beyond gastrointestinal protection.
Furthermore, the implications of Zhang’s findings extend to understanding the broader biological mechanisms underlying cellular responses to stress. By dissecting how natural compounds like 5-HMF can enhance resilience against radiation, the research also opens doors to exploring similar agents that may offer protective benefits in other contexts, such as in chemotherapy or severe inflammatory diseases.
It is crucial to acknowledge that while the results are promising, further clinical trials will be necessary to definitively establish efficacy and safety profiles for 5-HMF in human subjects. The pathway from laboratory discovery to clinical application is complex and requires rigorous validation to ensure that such treatments are both safe and effective.
The ongoing exploration into 5-HMF’s capacity to foster resilience in radiation-induced injuries aligns with a growing trend in integrative oncology that seeks to incorporate natural compounds into conventional treatment paradigms. This shift towards multifunctional approaches promises not only to improve patient quality of life but also to augment the efficacy of existing cancer therapies.
Zhang’s remarkable study brings forth a beacon of hope, illuminating a path that could lead to transformative advancements in cancer care. As research continues to shed light on the potential of low-dose 5-HMF, it significantly contributes to an evolving narrative that advocates for holistic and multifaceted treatments in oncology, addressing not just the cancer itself but also the myriad challenges faced by patients throughout their treatment journey.
In conclusion, the use of 5-HMF represents a strategic advance in oncological care and shines brightly as a potential game-changer in the mitigation of radiation-induced intestinal toxicity. As further investigations ensue, the ultimate goal remains clear: to enhance therapeutic effectiveness while safeguarding the well-being of patients navigating the complexities of cancer treatment.
Subject of Research: Radiation-induced intestinal toxicity and mitigation strategies.
Article Title: Low-dose 5-hydroxymethylfurfural mitigates radiation-induced intestinal toxicity via HIF2α-driven IL22/STAT3 signaling enhancement.
Article References:
Zhang, T., He, J., He, J. et al. Low-dose 5-hydroxymethylfurfural mitigates radiation -induced intestinal toxicity via HIF2α-driven IL22/STAT3 signaling enhancement. J Transl Med (2026). https://doi.org/10.1186/s12967-026-07757-3
Image Credits: AI Generated
DOI: 10.1186/s12967-026-07757-3
Keywords: 5-hydroxymethylfurfural, radiation therapy, intestinal toxicity, HIF2α, IL-22, STAT3, cancer treatment.
Tags: 5-HMF and tissue repaircancer treatment side effectsenhancing intestinal resiliencegastrointestinal tract damageHIF2α IL22/STAT3 signalinginflammation and cell apoptosisinnovative cancer treatment strategiesintestinal barrier protectionlow-dose 5-hydroxymethylfurfuralnatural compounds in cancer therapyradiation-induced intestinal toxicitytherapeutic interventions for radiation damage