In a groundbreaking study set to transform the therapeutic landscape for movement disorders, researchers have unveiled new insights into how visual states influence adaptive deep brain stimulation (aDBS) feedback signals. This innovative work, spearheaded by Zhu, GY., Merk, T., Butenko, K., and colleagues, delves deep into the neurophysiological mechanisms that underpin aDBS technologies, presenting a nuanced understanding of brain signal modulation that could revolutionize treatments for conditions like Parkinson’s disease.
Adaptive deep brain stimulation represents a significant advancement over traditional DBS therapies by adjusting electrical stimulation in real-time, responding dynamically to the brain’s fluctuating neural activity. The latest research reveals that visual states — the brain’s varying sensory and perceptual contexts — profoundly affect the feedback signals used to calibrate these stimulations. This discovery positions aDBS on a new frontier, one that integrates sensory environment considerations to optimize therapeutic outcomes.
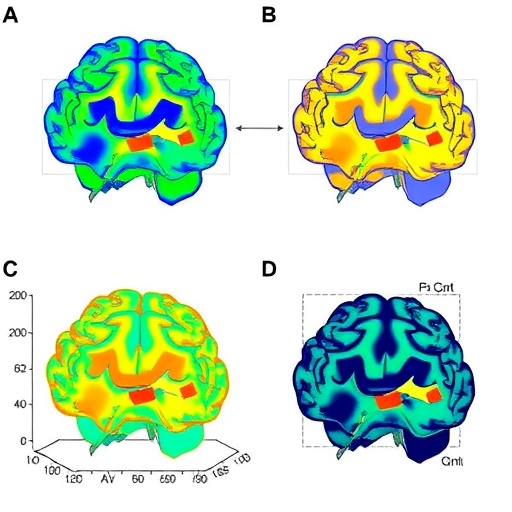
The technological premise of aDBS relies on complex closed-loop systems equipped with sensors capable of detecting specific brain biomarkers. These biomarkers serve as feedback signals, guiding the device to modulate stimulation parameters precisely, which helps to alleviate symptoms without inducing unwanted side effects. However, the research team identified that these signals are not solely influenced by motor states but also by the brain’s visual processing states, introducing a critical variable previously underappreciated in neurostimulation paradigms.
To investigate the intricate interplay between visual states and feedback signals, the researchers employed advanced neuroimaging techniques and electrophysiological recordings. Participants diagnosed with movement disorders underwent rigorous testing under varying visual conditions—ranging from completely darkened environments to complex visual scenes. The study revealed that changes in visual input altered the amplitude and frequency of the neural signals captured, directly impacting the aDBS adaptation algorithms.
These findings underscore the brain’s dynamic network connectivity, where visual stimuli modulate sensorimotor circuits. Such modulation affects local field potentials (LFPs), which are integral to the closed-loop feedback systems. By decoding how these LFPs shift according to visual context, the research opens avenues for more sophisticated algorithmic models that can accommodate environmental sensory inputs, thus tailoring stimulation more accurately to patient-specific needs and sensory environments.
One of the most compelling implications of this research is the potential to mitigate the variability in aDBS effectiveness that clinicians have observed in real-world settings. Patients often experience fluctuations in symptom relief correlated with changes in their ambient sensory conditions, which this study now explains at a neural circuit level. Integrating visual state considerations into stimulation protocols promises to stabilize therapeutic responses and improve quality of life significantly.
The study also advances the fundamental neuroscience understanding of cortico-basal ganglia-thalamo-cortical loops, highlighting how visual sensory processing intertwines with motor control pathways affected by Parkinson’s disease and other movement disorders. This neural crosstalk elucidates why static models of DBS feedback fail to capture the full spectrum of neural states, supporting the transition toward multimodal input-driven adaptive systems in next-generation neurostimulation devices.
Building on these insights, the researchers propose novel computational frameworks that utilize machine learning to interpret complex feedback patterns influenced by multifaceted sensory conditions. These frameworks can predict an optimal stimulation regime by incorporating not just motor-related signals but simultaneously accounting for visual state variables. This paradigm shift from unidimensional to multidimensional feedback models marks a new era in precision neuromodulation.
Moreover, the therapeutic electrode systems were fine-tuned to be sensitive to subtle shifts in visual state-evoked potentials, enabling the devices to preemptively adjust stimulation before motor symptoms exacerbate. This anticipatory modulation stands to reduce latency in response times, a critical factor in managing rapid fluctuations in disease severity. Such responsiveness promises to enhance the safety profile of aDBS by minimizing overstimulation risks.
The implications for patient-centric care are profound. By contextualizing stimulation parameters within the comprehensive sensory milieu of patients, aDBS systems could move beyond a one-size-fits-all approach. Personalized neural feedback profiles would not only improve motor symptom management but could also mitigate cognitive and affective side effects that arise from discordant sensory-motor integration during therapy.
This research sets a precedent for incorporating sensory neuroengineering into clinical neuromodulation strategies. It challenges existing clinical protocols by suggesting that sensory environment assessments should be integral to DBS programming and follow-up procedures. Such a holistic approach could become a cornerstone of future clinical guidelines, enhancing both efficacy and patient adherence to neurostimulation therapies.
In addition to Parkinson’s disease, these findings bear relevance for other movement disorders like dystonia and essential tremor, where sensory states might similarly influence stimulation outcomes. Longitudinal studies are anticipated to explore how sustained modulation of visual-evoked input affects disease progression and neuroplasticity, potentially uncovering new biomarkers for therapy optimization.
The translational potential of this work extends to the design of next-generation neuroprosthetics. By embedding adaptive feedback systems that interpret multimodal neural inputs, these devices could seamlessly integrate with the brain’s natural processing rhythms. This synthesis of technology and biology could ultimately restore motor functions with unprecedented fluidity and precision.
As artificial intelligence and neural interface technologies converge, the integration of sensory state decoding into aDBS heralds a future where brain-machine interfaces operate with intuitive adaptability. Such intelligent neurotherapeutics promise to transform the patient experience, ushering in an era where neurological impairment is met with highly responsive, context-aware interventions.
The study, published in the forthcoming issue of npj Parkinson’s Disease, represents a landmark advance in neuromodulation science. By decoding the impact of visual states on adaptive stimulation feedback, Zhu and colleagues have laid the groundwork for a new class of smart, sensory-informed neurostimulation therapies that hold tremendous promise for enhancing the lives of millions living with movement disorders worldwide.
Subject of Research: The neurophysiological influence of visual states on adaptive deep brain stimulation feedback signals in movement disorders, focusing on Parkinson’s disease.
Article Title: Decoding the impact of visual states on adaptive deep brain stimulation feedback signals in movement disorders.
Article References:
Zhu, GY., Merk, T., Butenko, K. et al. Decoding the impact of visual states on adaptive deep brain stimulation feedback signals in movement disorders. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-026-01273-3
Image Credits: AI Generated
Tags: adaptive deep brain stimulationbrain biomarkers for stimulation calibrationclosed-loop deep brain stimulation systemsdynamic electrical stimulation technologiesfeedback signals in deep brain stimulationinnovative approaches in neurotherapeuticsneurophysiological mechanisms of aDBSoptimizing therapeutic outcomes in movement disordersParkinson’s disease treatment advancementsreal-time brain signal modulationsensory influence on brain stimulationvisual states and neurophysiology