In groundbreaking research presented by a team led by Han, F., Xu, Y., and Qian, C., significant strides have been made in understanding the multifaceted role of GW4869 in the context of glioblastoma—a notoriously aggressive brain tumor. Utilizing advanced imaging techniques such as positron emission tomography (PET) with ^18F-FDG, the researchers provide compelling evidence that GW4869, a nontoxic inhibitor of exosome production, exhibits the potential to suppress malignant progression while also reversing the resistance of glioblastoma cells to temozolomide (TMZ), a standard chemotherapeutic agent.
Central to this study is the acknowledgment that glioblastoma poses an urgent challenge to oncologists worldwide due to its heterogeneity, treatment resistance, and poor prognosis. With a median survival rate often less than two years after diagnosis, researchers are racing to identify new therapeutic strategies. The role of tumor metabolism has emerged as a crucial factor, and this study examines how GW4869 may influence glucose metabolic phenotypes in glioblastoma.
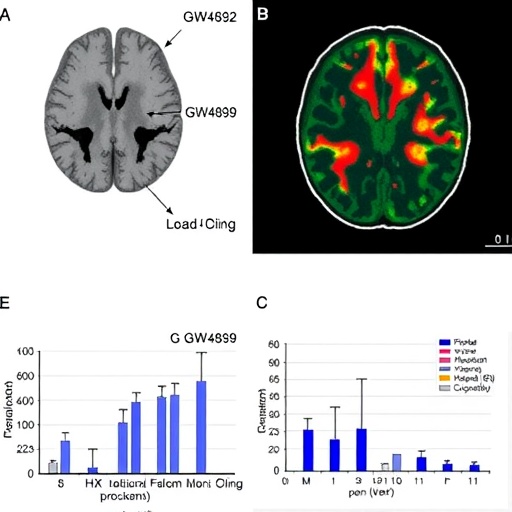
The innovative use of ^18F-FDG PET imaging allows for a detailed exploration of glucose uptake in tumor tissues, giving insight into the metabolic changes induced by GW4869. This imaging technique has become a cornerstone in cancer research, offering real-time data on metabolic activity that correlates with tumor burden and aggressiveness. The researchers demonstrate that GW4869 significantly alters glucose metabolism within glioblastoma cells, enhancing the understanding of how manipulating tumor metabolism can lead to improved outcomes.
Upon administration of GW4869, notable alterations were observed in the glucose metabolic pathways of glioblastoma cells. The authors reported a decrease in aerobic glycolysis, disrupting the Warburg effect—a hallmark of cancer cell metabolism characterized by increased glucose uptake and lactate production irrespective of oxygen availability. By counteracting this metabolic reprogramming, GW4869 may catalyze a shift towards more oxidative phosphorylation—an energy-generating process linked with better cellular health and reduced malignancy.
Moreover, the study importantly addresses the ongoing challenge of TMZ resistance in glioblastoma therapy. Many tumors develop adaptive responses that allow them to escape the cytotoxic effects of chemotherapy. The findings indicate that GW4869 not only mitigates cell proliferation but also enhances the sensitivity of glioblastoma cells to TMZ. This revelation opens the door for combination therapies that leverage GW4869’s effects to sensitize tumors that previously exhibited resistance.
Elucidating the mechanisms through which GW4869 achieves its anti-cancer effects, the researchers delved into the role of exosomes—small extracellular vesicles involved in intercellular communication and the transfer of oncogenic signals. By inhibiting exosome production, GW4869 effectively disrupts the tumor microenvironment’s ability to foster growth and survival, thereby suppressing the aggressiveness of glioblastoma. This mechanism suggests that targeting exosome release could be a novel strategy for curtailing glioblastoma progression.
To further validate these findings, in vivo experiments using glioblastoma animal models were conducted, reinforcing the therapeutic potential of GW4869 in clinical settings. Mice treated with GW4869 exhibited remarkable reductions in tumor size compared to controls. These promising results, displayed with the aid of PET imaging, underscore the necessity of rigorous clinical trials to evaluate GW4869’s efficacy and safety in human patients.
The overarching implications of this study are profound, suggesting a paradigm shift in how glioblastoma might be treated. By reprogramming metabolic pathways and enhancing response to existing chemotherapeutic agents, GW4869 presents a dual approach to combatting this formidable disease. As researchers continue to unravel the complexities of glioblastoma, the insights gleaned from this study may serve as a catalyst for developing novel therapeutic interventions.
Moreover, the findings draw attention to the larger context of cancer metabolism research. Manipulating metabolic pathways is gaining recognition as a crucial avenue for targeting advanced and resistant tumors. This study’s insights not only contribute to glioblastoma research but also have broader implications for understanding and treating other malignancies characterized by similar metabolic dysregulations.
This research catalyzes further inquiries into the intersection of exosome biology and tumor metabolism, paving the way for future studies aimed at leveraging this knowledge for therapeutic benefit. As the scientific community continues to probe the intricate mechanisms that underlie cancer progression, the hope remains that studies such as this will foster innovative approaches to improve patient outcomes in the challenging landscape of glioblastoma treatment.
In conclusion, the compelling findings reported by Han, F. and colleagues provide a significant leap forward in glioblastoma research, offering a multifactorial strategy not only for combating tumor aggressiveness but also for reversing treatment resistance. As the battle against this deadly disease unfolds, GW4869 offers a glimpse of hope that with continued investigation and refinement, effective therapies can emerge to prolong and enhance the quality of life for patients facing this daunting diagnosis.
Subject of Research: The suppression of glioblastoma progression and reversal of TMZ chemoresistance through GW4869.
Article Title: GW4869’s suppression of glioblastoma malignant progression and reversal of TMZ chemoresistance via glucose metabolic phenotype remodeling.
Article References:
Han, F., Xu, Y., Qian, C. et al. 18F-FDG PET imaging reveals GW4869’s suppression of glioblastoma malignant progression and reversal of TMZ chemoresistance via glucose metabolic phenotype remodeling. J Transl Med (2026). https://doi.org/10.1186/s12967-025-07668-9
Image Credits: AI Generated
DOI: 10.1186/s12967-025-07668-9
Keywords: Glioblastoma, GW4869, TMZ resistance, Metabolic reprogramming, Exosomes, ^18F-FDG PET imaging.
Tags: advanced imaging techniques in canceraggressive brain tumor challengeschemoresistance in brain tumorsexosome production inhibitionglioblastoma treatment strategiesglucose uptake in cancer therapyGW4869 glioblastoma researchmalignant progression suppressiononcological therapeutic innovationsPET imaging in oncologytemozolomide effectivenesstumor metabolism in glioblastoma