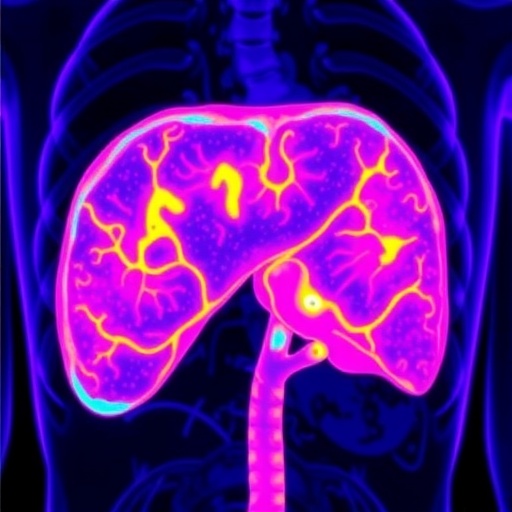
In the realm of surgical oncology, precise tumor delineation is paramount for successful intervention, especially during liver surgeries where the distinction between healthy and cancerous tissue can be particularly intricate. Current imaging techniques often fall short in terms of providing real-time, reliable guidance. A recent study presents an innovative approach, leveraging the unique properties of endogenous substances found within human liver tissues. This exciting advancement uncovers the utilization of intense autofluorescence in the second near-infrared window (NIR-II, 1,000–1,700 nm), facilitating a breakthrough in visualizing liver malignancies during surgical procedures.
The identification of these autofluorescent substances sets the stage for the development of a novel imaging technique known as tissue autofluorescence NIR-II imaging (TANI). Unlike conventional imaging modalities, TANI operates without the need for exogenous contrast agents, thus promising to enhance the safety and efficiency of liver surgeries. This non-invasive method harnesses the inherent optical properties of tissues, providing surgeons with a powerful tool to improve intraoperative decision-making and outcomes.
Testing the capabilities of TANI revealed extraordinary contrast ratios, averaging at an impressive 7.69 ± 0.52. This remarkable contrast underscores TANI’s potential to effectively distinguish between various types of liver tumors, including hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and metastatic lesions, regardless of the patient’s underlying liver condition, be it cirrhotic or non-cirrhotic. The high sensitivity of the imaging method, pegged at 97.8%, and an equally striking specificity rate of 98.4% showcase TANI’s robust performance in real clinical scenarios.
In contrast to existing techniques that utilize fluorescence-guided surgery in the visible light spectrum or the first near-infrared window, TANI demonstrates a superior ability to delineate cancerous tissues with minimal interference from benign lesions, blood, or bile contaminants. This is particularly noteworthy as the presence of such contaminants often complicates surgical procedures and leads to diagnostic challenges. The findings suggest a paradigm shift in how surgeons will approach liver surgeries, providing them with critical information that could alter the course of treatment on the spot.
Another significant advantage of TANI is its resilience against variations in cancer grade or stage. This consistency is essential as it underscores the technique’s reliability across a spectrum of liver malignancies, making it a valuable asset in the toolkit of contemporary surgical practices. Furthermore, the imaging capability is expected to enhance the surgeon’s ability to carry out oncological resections with greater precision, thereby reducing the likelihood of tumor recurrence post-surgery.
What truly elevates TANI as a groundbreaking application is its label-free nature. Surgeons can now rely on real-time imaging without administering any additional contrast agents, which could pose an extra risk to patients. This attribute aligns perfectly with the evolving focus towards patient-centric and minimally invasive surgical techniques that prioritize safety and effective patient outcomes.
The innovation presented by TANI not only holds promise for liver surgeries but may also extend its utility in other domains of oncology. The inherent autofluorescence properties observed may serve as a springboard for further research into other solid tumors. Indeed, the study hints at a strong correlation between near-infrared autofluorescence and various malignancies, inviting future inquiries into its applications across different tissue types and cancer forms.
Moreover, the opportunity to uncover molecular insights behind these autofluorescent substances could herald new diagnostic avenues. Understanding the biochemical nature of these substances may unlock additional layers of data crucial for distinguishing diseased tissues from healthy ones, possibly paving the path for the development of targeted therapies or new imaging biomarkers.
The promise of TANI as a revolutionary intraoperative management tool for liver cancer adds an exciting chapter to the ongoing pursuit of better diagnostic and therapeutic processes in oncology. As the medical community increasingly desires quick and reliable methodologies that lead to enhanced surgical performance, TANI stands as a testament to what innovative imaging technology can achieve.
With TANI proving its efficacy, the expectation is that implications will stretch far beyond the operating room, potentially influencing preoperative planning and overall cancer treatment strategies. When seamlessly integrated into surgical workflows, this technology may alter how liver malignancies are approached both from the surgical and the oncological perspective.
As researchers continue to explore the capabilities and expand the applications of TANI, one can anticipate a ripple effect across various medical specialties, leading to improvements in surgical techniques, enhanced patient safety, and ultimately, better clinical outcomes for individuals battling liver cancer.
To conclude, the advancements introduced through tissue autofluorescence NIR-II imaging represent a notable leap forward in the pursuit of precision medicine. As surgical practices continue to innovate and integrate groundbreaking technologies, the potential of TANI to reshape surgical oncology cannot be underscored enough. With promising clinical performance and strong foundational science, TANI is poised to set new standards in tumor visualization.
Subject of Research: Development of tissue autofluorescence NIR-II imaging (TANI) for visualization of human liver malignancies.
Article Title: Label-free tissue NIR-II autofluorescence imaging for visualization of human liver malignancy.
Article References:
He, H., Zhu, W., Miao, H. et al. Label-free tissue NIR-II autofluorescence imaging for visualization of human liver malignancy.
Nat. Biomed. Eng (2026). https://doi.org/10.1038/s41551-025-01593-4
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41551-025-01593-4
Keywords: liver malignancies, TANI, NIR-II imaging, surgical oncology, autofluorescence, intraoperative imaging, precision medicine, tumor visualization, cancer diagnosis, real-time imaging.
Tags: contrast ratios in medical imagingendogenous optical properties in tissuesenhancing surgery safety and efficiencyhepatocellular carcinoma detectionintraoperative decision-making toolsliver surgery innovationsliver tumor delineation techniquesNIR-II imaging technologynon-invasive cancer imaging methodsreal-time tumor visualizationsurgical oncology advancementstissue autofluorescence imaging