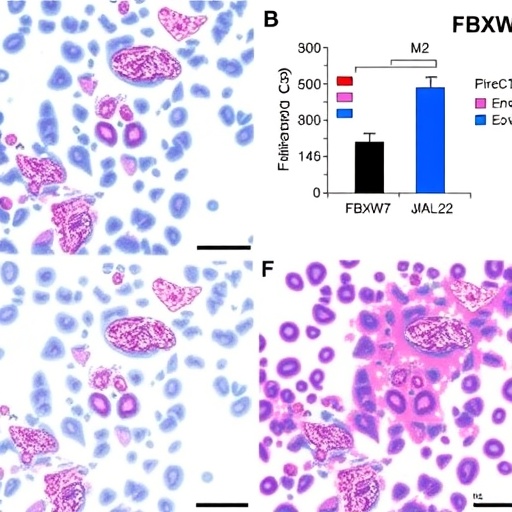
In the intricate landscape of cancer biology, the role of the tumor microenvironment is gaining increasing recognition. One of the pivotal components within this environment is the presence of immune cells, particularly macrophages, which can adopt various polarization states depending on the stimuli they encounter. In a recent breakthrough study by Wu, Zhang, and Xu et al., the focus is placed on the influence of the FBXW7 gene on M2 macrophage polarization in endometrial cancer, shedding light on the complex interplay between tumor cells and their immune counterparts.
FBXW7, or F-box and WD repeat domain-containing 7, is a crucial component of the ubiquitin-proteasome system, responsible for targeting specific proteins for degradation. The researchers explored how FBXW7 participates in regulating the immune landscape in endometrial cancer by specifically examining its role in the polarization of macrophages, particularly the M2 subtype, which is associated with tumor progression and a suppressive immune environment. This study provides valuable insights that could lead to the development of novel therapeutic strategies aimed at reprogramming macrophages in cancer therapies.
The polarization of macrophages into M2 phenotypes is often driven by the presence of certain cytokines and chemokines, one of the key players being CCL2 (C-C motif chemokine ligand 2). CCL2 is known for its ability to recruit monocytes to sites of tissue injury and inflammation, and its elevated levels in the tumor microenvironment can significantly influence tumor growth. The researchers found that FBXW7 negatively regulates the secretion of CCL2, thereby presenting a fascinating mechanism through which tumor cells might evade immune detection and promote their survival.
Interestingly, the study dives deep into the molecular mechanisms underlying FBXW7’s regulation of CCL2 secretion. The team discovered that FBXW7 targets MYBL2 for ubiquitination, a process that leads to the degradation of this transcription factor, thus inhibiting CCL2 production. MYBL2 is involved in regulating various cellular processes, including proliferation and differentiation, and its modulation by FBXW7 could have profound implications for tumor-associated macrophage dynamics.
This research also emphasizes the importance of post-translational modifications in the regulation of gene expression within the tumor microenvironment. By elucidating how FBXW7 acts as a key controller of MYBL2, the study provides a potential link between ubiquitination processes and the modulation of cytokine secretion in endometrial cancer. The findings suggest that targeting the FBXW7-MYBL2 axis could be a novel approach for reshaping immune responses against tumors, potentially transforming the therapeutic landscape for patients battling this type of cancer.
Moreover, the intricate relationship between tumor cells and macrophages is further highlighted by examining the broader implications of the study. With M2 macrophages not only facilitating tumor growth but also modulating the immune response, the ability to manipulate their polarization could pave the way for innovative cancer therapies. Inhibiting M2 polarization may lead to a more robust anti-tumor immune response, thus enhancing the efficacy of existing treatments or leading to the development of new ones.
As researchers continue to unravel the complexities of tumor-immune interactions, the FBXW7/MYBL2 pathway represents a promising therapeutic target. The call for more research in this area is critical, as understanding the mechanistic pathways that drive macrophage polarization could unveil entirely new strategies for cancer immunotherapy. The potential to convert M2 macrophages back to a more anti-tumorigenic M1 state is enticing, heralding a new age of targeted therapies.
The significance of these findings goes beyond the immediate implications for endometrial cancer; they offer insights applicable to various other cancers where M2 macrophage polarization plays a detrimental role. As such, continued investigation into this pathway could have far-reaching consequences, providing a framework for future studies aimed at harnessing the immune system’s power to combat neoplastic diseases.
In conclusion, the research conducted by Wu, Zhang, and Xu et al. marks a pivotal step forward in our understanding of macrophage polarization in the context of endometrial cancer. By identifying FBXW7 as a critical regulator of CCL2 secretion and its downstream effects on MYBL2, the study opens up new avenues for therapeutic intervention. The potential to target and reprogram the immune landscape offers exciting possibilities for improving patient outcomes and redefining treatment paradigms in cancer therapy.
As we stand at the crossroads of cancer research and immunology, it becomes increasingly clear that the intricate relationships between cancer cells and immune components are crucial to developing more effective treatments. With each discovery, such as the role of FBXW7 in macrophage polarization, we move closer to unlocking the secrets of the tumor microenvironment and enhancing our arsenal against cancer.
With continued exploration and validation of these mechanisms, we can hope to transition from understanding the basic biology of cancer to applying this knowledge in therapeutic settings, ultimately reducing the burden of cancer on patients and society at large. The work of Wu, Zhang, and Xu et al. exemplifies the importance of academic inquiry in this ever-evolving field and inspires further research endeavors that seek to combat the scourge of cancer through innovative and informed approaches.
Subject of Research: Macrophage polarization in endometrial cancer
Article Title: FBXW7 Inhibited M2 Macrophage Polarization in Endometrial Cancer by Reducing CCL2 Secretion Through Ubiquitination of MYBL2 Subtitle: The Role of FBXW7 on M2 Macrophage Polarization in EC
Article References: Wu, J., Zhang, X., Xu, W. et al. FBXW7 Inhibited M2 Macrophage Polarization in Endometrial Cancer by Reducing CCL2 Secretion Through Ubiquitination of MYBL2 Subtitle: The Role of FBXW7 on M2 Macrophage Polarization in EC. Biochem Genet (2026). https://doi.org/10.1007/s10528-025-11309-7
Image Credits: AI Generated
DOI: https://doi.org/10.1007/s10528-025-11309-7
Keywords: endometrial cancer, FBXW7, macrophage polarization, CCL2, MYBL2, ubiquitination, tumor microenvironment, immunotherapy.
Tags: cancer immunology and macrophage interactionCCL2 cytokine influence on macrophagescytokines and chemokines inendometrial cancer immune landscapeFBXW7 gene role in endometrial cancerimmune modulation in cancer therapyM2 macrophage polarization mechanismsmacrophage polarization in cancer progressiontherapeutic strategies for macrophage reprogrammingtumor microenvironment and immune cellstumor-associated macrophages in endometrial cancerubiquitin-proteasome system in cancer