In recent years, the field of pharmacology has witnessed a transformative shift towards integrative approaches that enhance our understanding of complex biological systems. One notable study exemplifying this trend is conducted by researchers led by Yang et al., where they delve into the therapeutic potential of formononetin, a bioactive compound found in traditional Chinese medicine, particularly in Huangqin decoction. This multidimensional research combines network pharmacology with molecular docking, providing insights into the anti-inflammatory properties of formononetin as validated through experiments in a zebrafish model of inflammatory bowel disease (IBD).
The study begins by contextualizing the relevance of Huangqin decoction, a formulation that has been utilized for centuries to ameliorate various ailments, particularly those characterized by inflammation. Its ingredients have been widely acknowledged for their synergistic effects, and formononetin, in particular, has emerged as a molecule of interest due to its potential benefits in managing inflammatory responses. The investigation seeks to unravel the mechanistic pathways through which formononetin exerts its effects, thereby contributing to the broader discussions surrounding integrative and complementary medicine.
To approach this inquiry, Yang and colleagues employed network pharmacology, an innovative technique that maps the interactions between drugs and biological systems. This approach allows researchers to identify not only the primary targets of a compound but also its broader biological implications. In the context of formononetin, the study elucidates the intricate network of protein-ligand interactions, identifying key molecular targets that mediate its anti-inflammatory effects. This systematic analysis is essential for bridging the gap between traditional knowledge and modern scientific validation.
Following the network pharmacology analysis, the research team utilized molecular docking simulations to further investigate the interactions between formononetin and its identified targets. Molecular docking is a computational technique that predicts the preferred orientation of a molecule when bound to a target protein, thereby revealing potential binding affinities. The findings from this phase of the study underscore formononetin’s ability to engage with inflammatory signaling pathways, highlighting its potential to inhibit pro-inflammatory cytokines and modulate immune responses.
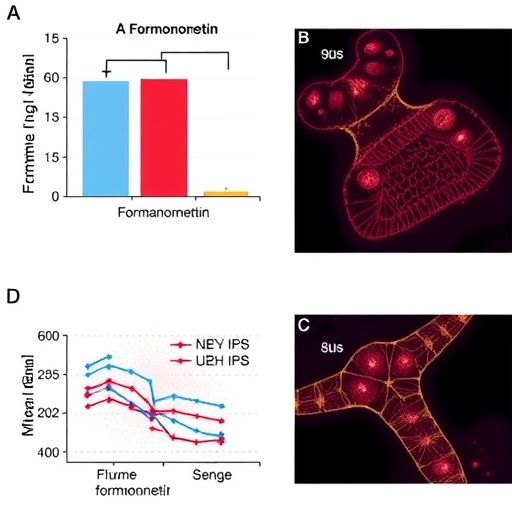
To substantiate their computational findings, Yang et al. proceeded to conduct experiments on a zebrafish model of DSS-induced IBD. This model is particularly useful due to the similarities in the inflammatory processes between zebrafish and humans, allowing for the effective assessment of therapeutic outcomes. The experiments confirmed that administration of formononetin resulted in significant alleviation of inflammatory symptoms, showcasing a decrease in intestinal damage and an improvement in overall health indicators in the zebrafish subjects.
The implications of these findings are manifold. The study not only reinforces the therapeutic potential of formononetin as an anti-inflammatory agent but also advocates for the importance of integrating traditional medicines into contemporary therapeutic frameworks. This research serves as a critical reminder of the rich pharmacological wisdom embedded in traditional herbal remedies and emphasizes the need for rigorous scientific inquiry to validate these practices.
Moreover, the study opens avenues for further research into the applications of formononetin in other inflammatory conditions, potentially extending its benefits to a broader range of diseases that plague human health. The success of this research exemplifies how modern techniques such as network pharmacology can effectively unravel the complexities surrounding herbal components, fostering an environment for innovative therapeutic strategies in inflammatory diseases.
The comprehensive nature of the investigation—spanning computational analyses and empirical validation—demonstrates an orderly progression from hypothesis generation to experimental confirmation. This methodology is paramount in establishing the credibility of findings and ensuring that traditional medicine is not relegated to anecdotal efficacy but is instead viewed through the lens of modern scientific rigor.
As the conversation around the integration of complementary and alternative medicine into mainstream healthcare continues to grow, studies like that conducted by Yang et al. are pivotal. They not only provide evidence-based recommendations for the use of traditional therapies but also contribute to a more holistic understanding of health that transcends the limitations of single-target approaches.
In conclusion, the integration of network pharmacology, molecular docking, and empirical validation in the study of formononetin represents a significant stride towards understanding the intricacies of herbal medicine. As more researchers embrace this integrative approach, it paves the way for novel therapeutic discoveries that could redefine treatment paradigms for various chronic conditions, especially inflammatory diseases.
The research conducted by Yang and colleagues shines a light on an often-overlooked avenue of pharmacological exploration and reaffirms the potential of combining traditional knowledge with advanced scientific techniques. Given the growing global burden of chronic inflammatory diseases, their findings hold promise for advancing therapeutic options through a blend of ancient wisdom and modern science.
As healthcare continues to evolve, embracing practices that leverage historical knowledge while integrating cutting-edge technology will be crucial in addressing future health challenges. This study not only contributes valuable insights into the pharmacological potential of formononetin but also advocates for a more inclusive understanding of health that honors both tradition and innovation in equal measure.
In summary, the integration of network pharmacology and molecular docking with experimental validations in this study underscores a promising direction for future research. The implications of these findings reach far beyond the initial scope, potentially affecting clinical practices and offering new hope for patients suffering from inflammatory diseases.
Subject of Research: Anti-inflammatory efficacy of formononetin in Huangqin decoction.
Article Title: Integration of network pharmacology and molecular docking reveals the anti-inflammatory efficacy of formononetin in Huangqin decoction and experiment verification in DSS-induced zebrafish IBD model.
Article References: Yang, X., Tang, Q., Dou, J. et al. Integration of network pharmacology and molecular docking reveals the anti-inflammatory efficacy of formononetin in Huangqin decoction and experiment verification in DSS-induced zebrafish IBD model. BMC Complement Med Ther 25, 450 (2025). https://doi.org/10.1186/s12906-025-05188-z
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12906-025-05188-z
Keywords: formononetin, Huangqin decoction, network pharmacology, molecular docking, anti-inflammatory, zebrafish model, inflammatory bowel disease, traditional medicine.
Tags: bioactive compounds in pharmacologycomplementary medicine strategiesformononetin anti-inflammatory effectsHuangqin decoction traditional medicineinflammatory bowel disease IBD treatmentintegrative medicine approachesmechanistic pathways of inflammationmolecular docking in drug discoverynetwork pharmacology applicationspharmacological research innovationstherapeutic potential of formononetinzebrafish model in research