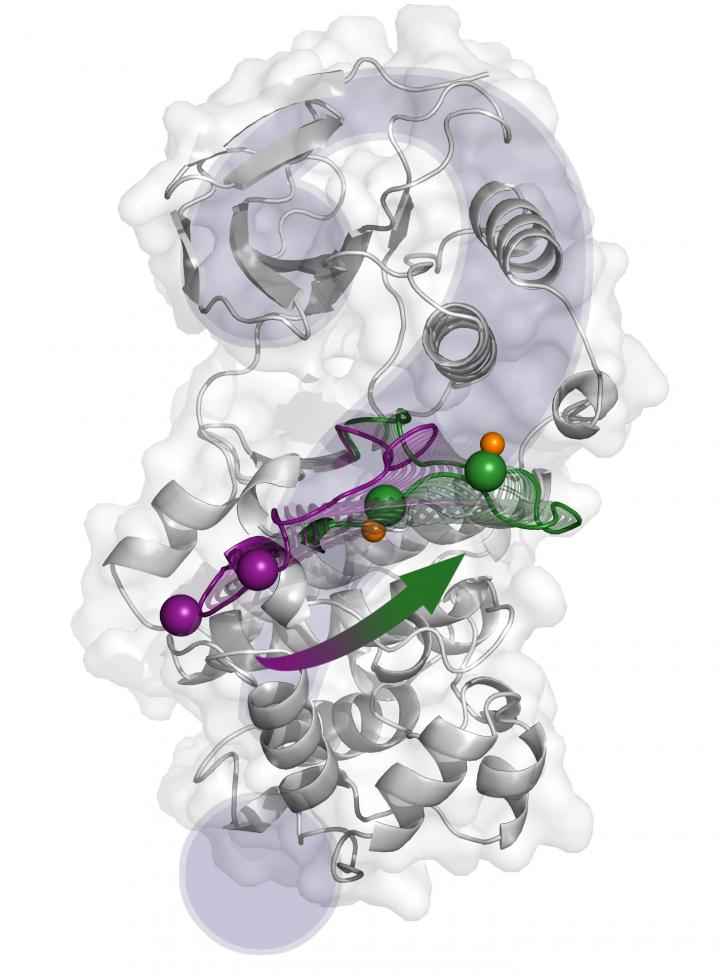
Credit: Antonija Kuzmanic.
The protein p38α is a member of a family of molecules that transmit outside signals throughout the cell, thus allowing for an appropriate cell response, such as proliferation, differentiation, senescence, or death. Moreover, the participation of p38α in pathological conditions, like chronic inflammatory diseases and cancer, makes it a promising pharmacological target. In this regard, a complete picture of the activation mechanism of this protein is essential in order to design specific inhibitors that do not affect other processes.
The journal eLife has published a study on p38α by Antonija Kuzmanic, an EU Marie Curie COFUND fellow who is undertaking postdoctoral training simultaneously in two IRB Barcelona labs — the Molecular Modelling and Bioinformatics Laboratory and the Signalling and Cell Cycle Laboratory. Collaborative research between the lab headed by Modesto Orozco and that led by Angel R. Nebreda, the latter an international authority on p38α, has provided an integrative picture of the p38α activation mechanism and new insights into the molecular effects of various molecules that regulate the enzymatic activity of the protein.
Using computational techniques, researchers have deciphered the key elements of the complex molecular mechanism underlying p38α activity. This study describes the protein activation mechanism in unprecedented detail and reconciles the apparent contradictory results reported in previous structural studies. "Considering the importance of p38α for pathological processes, we hope the knowledge obtained in this study will help to target the protein with more specificity," stresses Antonija Kuzmanic, first author of the study.
Identifying new inhibitors
p38α has already been targeted for inflammatory diseases and some types of cancer; however, none of the drugs have yet made it to the market. "Our study reveals novel conformations of the protein, which could be used as a starting point in virtual screening studies aimed at uncovering new inhibitors," explains Kuzmanic. And she adds, "We were also able to highlight important electrostatic interactions, which may allow us to explore alternative activation pathways with increased specificity".
A computational biology approach
"We used only computational techniques. Mainly, we employed numerous molecular dynamics simulations combined with an advanced sampling technique called metadynamics," explains Kuzmanic. This combination has an advantage over standard molecular dynamics simulations, as it allows researchers to observe large conformational changes in a reasonable amount of computational time. She goes on to say, "we are able to add statistical significance to the conformations we observed in our simulations".
###
Reference article:
Antonija Kuzmanic, Ludovico Sutto, Giorgio Saladino, Angel R. Nebreda, Francesco L. Gervasio and Modesto Orozco.
Changes in the free-energy landscape of p38α MAP kinase through its canonical activation and binding events as studied by enhanced molecular dynamics simulations
eLife (2017) DOI: 10.7554/eLife.22175
Media Contact
Sònia Armengou
[email protected]
34-934-037-255
http://www.irbbarcelona.org
############
Story Source: Materials provided by Scienmag