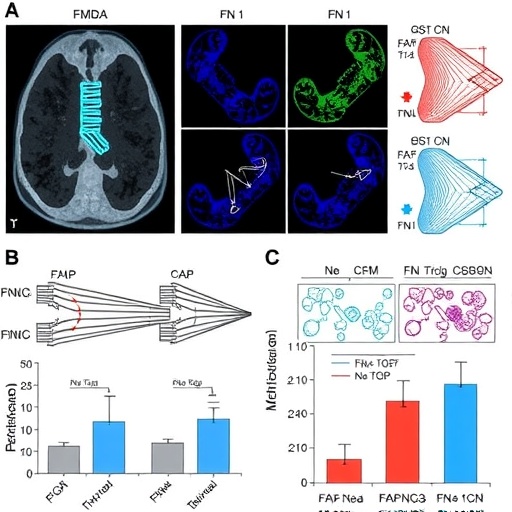
In a groundbreaking study poised to reshape the understanding of aggressive thyroid cancer, researchers have unveiled significant insights into the molecular mechanisms driving metastasis through the fibronectin 1-transforming growth factor beta (FN1-TGFβ) axis. This research elucidates the role of fibroblast activation protein (FAP) in promoting both tumor progression and immune evasion, marking a pivotal advancement in cancer biology.
The study, led by Udinotti and colleagues, meticulously explores the interactions within the tumor microenvironment that facilitate metastasis, a process whereby cancer cells spread from their origin to distant sites. The findings suggest that FAP plays a crucial role in enhancing the invasive potential of thyroid cancer cells, particularly those characterized by aggressive growth patterns. As the research highlights, understanding these pathways can open avenues for targeted therapies.
Thyroid cancer, despite being one of the less common forms of cancer, exhibits a concerning rise in incidence, particularly among younger populations. This increasing prevalence underscores the urgency for deeper investigations into the mechanisms that govern its aggressive forms. By focusing on the FN1-TGFβ axis, the researchers have identified a vital signaling pathway that orchestrates various cellular processes contributing to tumor metastasis.
FAP, a serine protease often associated with cancer-associated fibroblasts, has been implicated in modulating the tumor microenvironment and enhancing the tumor’s ability to evade immune surveillance. The current study underscores the dual role of FAP, not only in promoting tumor cell migration but also in facilitating immune suppression, thereby allowing the cancer to thrive and spread unchecked.
Immune suppression in cancer is a well-documented phenomenon, significantly complicating treatment strategies. The study reveals that thyroid cancer cells can manipulate immune responses to their advantage, creating a conducive environment for their metastasis. This manipulation occurs through the regulation of TGFβ, which is known to have profound effects on immune cell function, often skewing responses in favor of the tumor.
One of the highlights of the research is its potential to inform therapeutic strategies aimed at disrupting these pathways. By targeting the FAP-mediated processes, new treatments could be devised that not only inhibit tumor growth but also reestablish immune surveillance mechanisms. This offers a hopeful perspective for patients with aggressive thyroid cancer, who currently face limited effective treatment options.
Moreover, the implications of this research extend beyond thyroid cancer alone. The mechanisms revealed could be applicable to various solid tumors where FAP and the FN1-TGFβ axis play a role in metastasis. Hence, this work opens up a broad field for exploring similar pathways in other cancers, potentially leading to new therapeutic interventions across multiple cancer types.
In an age where personalized medicine is becoming the norm, understanding the genetic and molecular underpinnings of aggressive cancers is vital. The study by Udinotti et al. emphasizes the need for precision oncology approaches that tailor treatments based on specific molecular profiles rather than a one-size-fits-all strategy. This research exemplifies how dissecting the intricacies of tumor biology can pave the way for tailored therapies, ultimately improving patient outcomes.
Furthermore, given the increasing push for immunotherapies, the role of FAP and the FN1-TGFβ axis in immune evasion presents a compelling target for combination therapies. Integrating FAP inhibitors with existing immunotherapeutic agents could enhance the overall effectiveness of treatment regimens and re-sensitize tumors to immune-mediated destruction.
The findings also raise important questions about future directions in research. As scientists delve deeper into the interactions of the tumor microenvironment, investigating how other components, such as extracellular matrix proteins and immune cell types, influence cancer progression will be essential. The interplay between these factors could further illuminate strategies to disrupt the supportive networks that facilitate metastasis.
Patient advocacy groups and healthcare providers should take note of these developments, as they could influence patient management strategies in the near future. Engaging in dialogue about such research findings will be crucial as physicians strive to provide the best care for their patients diagnosed with aggressive thyroid cancer.
In summary, Udinotti and colleagues have significantly advanced the field of cancer research with their findings on FAP and the FN1-TGFβ axis in aggressive thyroid cancer. Their work not only elucidates critical mechanisms of metastasis and immune evasion but also lays the groundwork for future therapeutic innovations. The landscape of cancer treatment may soon be altered, offering hope to patients battling one of the more challenging forms of cancer.
This study serves as a reminder of the intricate relationships within cancer biology. As researchers continue to unravel these complex webs, the potential for breakthroughs that improve patient care and survival rates becomes increasingly feasible. Indeed, the fight against thyroid cancer—and cancer in general—may enter a new era of understanding and treatment.
Subject of Research: The role of fibroblast activation protein (FAP) in metastasis and immune suppression in aggressive thyroid cancer.
Article Title: Fibroblast activation protein (FAP)-mediated promotion of metastasis via the FN1-TGFβ axis and immune suppression in aggressive thyroid cancer.
Article References:
Udinotti, M., Siebolts, U., Bauer, M. et al. Fibroblast activation protein (FAP)-mediated promotion of metastasis via the FN1-TGFβ axis and immune suppression in aggressive thyroid cancer.
J Transl Med 23, 1284 (2025). https://doi.org/10.1186/s12967-025-07307-3
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12967-025-07307-3
Keywords: Fibroblast activation protein, thyroid cancer, metastasis, immune suppression, FN1-TGFβ axis.
Tags: aggressive thyroid cancer mechanismscancer progression pathwaysfibroblast activation protein role in cancerfibronectin 1-transforming growth factor beta axisimmune evasion in cancermolecular mechanisms of cancer metastasisrising incidence of thyroid cancersignaling pathways in tumor metastasistargeted therapies for thyroid cancerthyroid cancer metastasistumor microenvironment interactionsunderstanding thyroid cancer biology