In a groundbreaking study that promises to reshape our understanding of neural physiology, researchers have unveiled new insights into the cerebrospinal fluid (CSF) dynamics within the human brain using advanced magnetic resonance imaging (MRI) techniques. This work, recently published in Nature Neuroscience, marks a significant leap forward in deciphering the complex regional drivers that govern CSF mobility, shedding light on mechanisms that may underpin a variety of neurological diseases and potentially influence future therapeutic strategies.
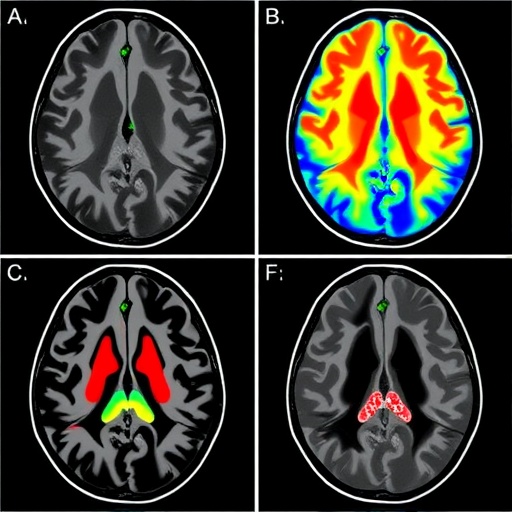
Central to this exploration is the investigation of how CSF—a clear, colorless body fluid found within the brain and spinal cord—moves through different regions of the brain. Historically, the movement of CSF has been challenging to characterize with precision in living humans due to limitations in non-invasive imaging modalities. The study harnesses a novel MRI-based approach that quantifies CSF velocity with unprecedented spatial resolution and sensitivity, allowing researchers to visualize the fluid’s intricate, region-specific flow patterns.
What stands out in this research is the emphasis on dissecting distinct anatomical areas, revealing that CSF motion is not a monolithic, uniform process. Rather, different brain regions exhibit unique driving forces influencing fluid dynamics, influenced by the microanatomy and vascular pulsatility that vary throughout the brain’s complex architecture. Such revelations position this study at the forefront of neuroimaging advances that move beyond static images toward dynamic, functionally relevant physiological mapping.
The team employed state-of-the-art phase-contrast MRI protocols, specifically optimized to capture subtle fluid velocities within the cranial cavity. These methods enable a pixel-by-pixel quantification of flow velocities, capturing oscillations synchronous with cardiac activity. This is crucial because heart-driven pulsations are understood to be major contributors to CSF movement, but the regional heterogeneity of their effect had remained elusive until now.
Focusing on a cohort of healthy volunteers, the investigators mapped CSF flow at multiple brain loci, including ventricular spaces, the subarachnoid compartments, and perivascular regions. Their quantitative data revealed that certain compartments exhibit pronounced flow signatures corresponding to cardiac and respiratory cycles, whereas others showed dampened or delayed responses. This spatiotemporal coupling between vascular rhythms and CSF movement offers compelling evidence for localized biomechanical interactions modulating fluid transport.
Beyond mapping normal physiology, the data provide a crucial reference framework for understanding pathological alterations. Since impaired CSF circulation is implicated in neurodegenerative disorders, such as Alzheimer’s disease, hydrocephalus, and multiple sclerosis, the identification of region-specific drivers of CSF mobility could unlock new diagnostic markers or therapeutic targets. For instance, aberrant flow patterns in the perivascular spaces might indicate early vascular or glymphatic system dysfunction, potentially preceding overt clinical symptoms.
Intriguingly, the study also touches upon the role of the brain’s glymphatic system—a recently characterized mechanism responsible for clearing metabolic waste and maintaining homeostasis. The authors propose that their regionally resolved CSF flow measurements might reflect glymphatic function at work, with implications for understanding how the brain self-cleans during sleep or following injury. By refining non-invasive biomarkers of glymphatic activity, this research could accelerate the development of interventions aimed at enhancing brain clearance mechanisms.
The interdisciplinary nature of this work, integrating expertise in neuroimaging, fluid dynamics, and brain physiology, underscores the complexity of CSF behavior. The use of MRI to capture dynamic physiological processes in vivo represents a transformative approach that could be extended to other bodily fluids and organ systems. The refinement of these imaging technologies is likely to catalyze a wave of studies exploring fluid mechanics in health and disease across a range of biomedical fields.
Critically, the study’s methodology addresses previous technical hurdles by combining advanced MR data acquisition with sophisticated modeling frameworks that account for pulsatile flow and tissue compliance. By tailoring imaging sequences to the temporal characteristics of cardiac-induced flow, the researchers maximized sensitivity to subtle velocity changes that were otherwise obscured in conventional scans. Moreover, the rigorous validation against physiological parameters adds robustness to the findings.
This research also opens the door to exploring how external interventions, such as pharmacologic agents or physical therapies, might modulate CSF flow regionally. Understanding the drivers of normal CSF mobility enables scientists and clinicians to hypothesize about potential manipulation strategies to restore or enhance fluid dynamics in patients suffering from CSF-related disorders. Such translational potential elevates the importance of these findings beyond basic science into clinical realms.
Moreover, the results challenge previously held notions about CSF circulation being predominantly passive or uniform. Instead, the findings support a paradigm in which localized forces, possibly mediated by vascular pulsatility or tissue elasticity, actively shape fluid transport pathways. This refined understanding has implications for computational modeling of brain fluid mechanics and for the interpretation of diagnostic imaging in neurological practice.
As fluid dynamics within the CNS become better delineated, there is growing interest in their broader physiological and pathological correlates. For example, the study’s insights could aid in unraveling the multifaceted interactions between CSF flow and intracranial pressure regulation, shedding light on conditions such as idiopathic intracranial hypertension or traumatic brain injury. By providing a map of normative CSF kinetics, deviations associated with these ailments may be better characterized.
The technological advancements driving this work are equally notable. Employing phase-contrast MRI as a non-invasive probe of brain fluid movement with such granularity requires both hardware precision and computational finesse. The integration of time-resolved imaging with cardiac gating techniques exemplifies the cutting edge of neuroimaging innovation, merging engineering and clinical insight to tackle longstanding neuroscientific questions.
Looking forward, this study sets the stage for longitudinal investigations monitoring how aging, disease progression, or therapeutic interventions alter CSF flow dynamics. By establishing baseline patterns in health, future research can identify early markers of dysfunction, enabling preemptive diagnostic approaches. Additionally, expanding these imaging protocols to larger and more diverse populations will help elucidate variability and normative ranges across demographic groups.
This pioneering endeavor not only enriches our understanding of CSF dynamics but also energizes a broader scientific dialogue about the interplay between brain structure, function, and fluid physiology. The ability to visualize and quantify these processes in vivo revolutionizes the potential for discovery and therapeutic innovation. As such, this work exemplifies the powerful synergy of advanced imaging, physiological modeling, and clinical neuroscience pushing the boundaries of what we know about our most vital organ.
In sum, the unveiling of region-specific drivers of CSF mobility reshapes classical views and opens exciting avenues for research and clinical application. With potential ramifications ranging from neurodegenerative disease diagnostics to novel treatment designs, this study exemplifies how precision imaging rapidly elevates our grasp of complex biological systems. As these MRI technologies become more accessible and refined, the coming years promise an explosive growth in our ability to monitor and manipulate brain fluid dynamics, paving the way for revolutionary neurological health care.
Subject of Research: Cerebrospinal fluid mobility and region-specific drivers of CSF dynamics in the human brain measured with MRI
Article Title: Region-specific drivers of CSF mobility measured with MRI in humans
Article References:
Hirschler, L., Runderkamp, B.A., Decker, A. et al. Region-specific drivers of CSF mobility measured with MRI in humans. Nat Neurosci (2025). https://doi.org/10.1038/s41593-025-02073-3
Image Credits: AI Generated
Tags: advanced imaging modalitiesanatomical variations in CSF movementcerebrospinal fluid dynamicshuman brain CSF flowmicroanatomy and vascular pulsatilityMRI techniquesNature Neuroscience studyneural physiology researchneurological disease mechanismsnon-invasive imaging advancementsregional drivers of CSF mobilitytherapeutic strategies for brain disorders