In a groundbreaking study poised to reshape our understanding of rare vascular emergencies, researchers Wei and Chen have employed patient-specific computational fluid dynamics (CFD) to unravel the complex hemodynamic landscape of spontaneous isolated superior mesenteric artery dissection (SISMAD). Published in BioMedical Engineering OnLine, this investigation delves deep into the intricate blood flow patterns and forces within the superior mesenteric artery when disrupted by dissection, presenting data that could revolutionize personalized diagnostics and treatment strategies for this elusive condition.
SISMAD, characterized by a tear in the superior mesenteric artery wall without accompanying aortic involvement, remains a mysterious and seldom-encountered clinical entity. The pathogenesis of this vascular rupture has long eluded clinicians, with hemodynamic stress posited as a pivotal yet insufficiently characterized contributor. The current study pioneers the use of three-dimensional patient-specific models reconstructed from high-resolution computed tomography angiography scans, offering unprecedented insight into the fluid dynamics shaping disease progression.
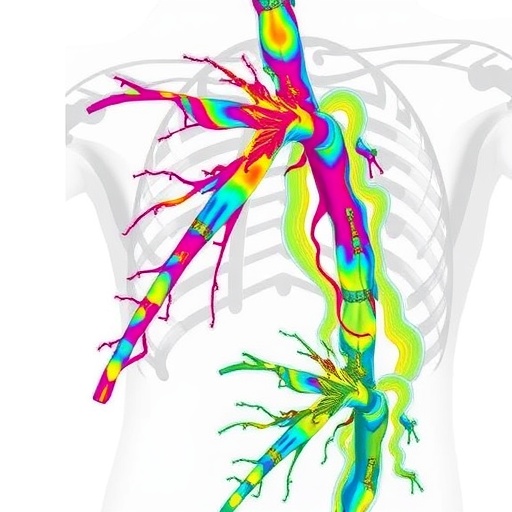
Central to the study’s methodology was the creation of detailed CFD simulations differentiating flow within the true lumen (TL)—the original arterial passageway—and the false lumen (FL), the novel cavity formed by the dissection plane. This approach allowed for precise quantification of velocity fields, wall shear stress (WSS), and oscillatory shear index (OSI), parameters known to influence vascular integrity and pathological remodeling. The dissected artery analyzed exhibited heterogenous flow phenomena that illuminate the biomechanical environment driving clinical outcomes.
.adsslot_awk1lUe7Fg{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_awk1lUe7Fg{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_awk1lUe7Fg{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The true lumen was observed to harbor high-velocity flow concentrated predominantly near the entry tear site, resulting in significantly elevated WSS and time-averaged wall shear stress (TAWSS) along the intimal flap. These intense forces implicate a potentially destabilizing mechanical aggression on the arterial wall, serving as a catalyst for flap propagation or rupture. Such detailed hemodynamic profiling affirms long-held suppositions regarding the correlation between localized shear stress and vascular wall degradation yet quantifies these forces with unmatched precision.
Contrastingly, the false lumen presented starkly different hemodynamics. Flow velocities were markedly reduced, with pronounced zones of stagnation and flow recirculation. This sluggish hemodynamic milieu corresponded with diminished WSS values, creating a permissive environment for thrombus formation. Elevated OSI values—reaching peaks of 0.45 within the false lumen—indicate an unstable and oscillatory flow directionality, conditions known to promote endothelial dysfunction and clotting cascades. This compelling evidence underscores the role of flow-induced factors in driving thrombogenesis within the dissected artery.
Additionally, the study identified a significant pressure gradient across the intimal flap separating the true and false lumens, with the true lumen consistently exhibiting higher pressure. This gradient likely influences both the mechanical stress on the dissection flap and the risk of flap progression, suggesting that localized hemodynamic forces contribute not only to acute events but also to chronic remodeling and potential occlusion. The quantification of such pressures marks a significant step forward in understanding mechanical triggers of dissection evolution.
By employing patient-specific CFD modeling, this research transcends traditional imaging limitations, offering a fluid mechanics perspective that integrates structural morphology with dynamic blood flow forces. The use of individualized arterial geometries ensures that the observed hemodynamics reflect true physiological conditions rather than generic assumptions, paving the way for personalized medicine applications in vascular pathology. This marks a paradigm shift from solely anatomical diagnoses to mechanistic evaluations encompassing biomechanical stresses.
Beyond theoretical advancements, the study’s findings have palpable clinical implications. The identification of regions subjected to elevated shear stress suggests potential targets for therapeutic intervention or monitoring, while the recognition of thrombogenic zones within the false lumen raises the potential for tailored anticoagulation strategies. Clinicians could leverage such detailed hemodynamic profiles to stratify patients by risk, guiding decisions about medical management versus invasive repair.
Moreover, the delineation of heterogeneous flow behaviors between true and false lumens illuminates the multifaceted nature of SISMAD pathophysiology. The interaction between high-stress regions prone to flap instability and low-flow zones susceptible to thrombus accumulation highlights the need for comprehensive treatment plans addressing both mechanical reinforcement and thrombosis prevention. These insights could culminate in improving patient outcomes through precision interventions.
The technology underpinning this work—patient-specific CFD—represents the vanguard of vascular research techniques. By integrating high-resolution imaging with advanced numerical modeling, researchers can simulate blood flow patterns under physiologically realistic conditions, capturing transient dynamics and spatial heterogeneity inaccessible through conventional diagnostics. As computational power and imaging modalities continue to advance, such approaches are anticipated to become routine tools in clinical vascular assessment.
Importantly, this study also contributes to the broader understanding of arterial dissections beyond SISMAD. The hemodynamic principles elucidated here regarding stress gradients, flow stasis, and oscillatory forces may have parallels in other dissecting vascular diseases, such as aortic or carotid dissections. Consequently, these findings may influence future research trajectories across multiple vascular territories, enhancing diagnostic and therapeutic frameworks.
In conclusion, Wei and Chen’s innovative application of patient-specific computational fluid dynamics offers a transformative glimpse into the hemodynamic intricacies of spontaneous isolated superior mesenteric artery dissection. By dissecting flow velocity, shear stresses, and pressure gradients within pathologically altered arterial lumens, their work bridges a critical knowledge gap between vascular biomechanics and clinical pathology. This mechanistic perspective not only deepens scientific understanding but also heralds a new era of individualized vascular care, where computational insights guide risk stratification and personalized therapy in a life-threatening yet enigmatic disease.
Subject of Research: Hemodynamic forces and flow characteristics in spontaneous isolated superior mesenteric artery dissection (SISMAD) analyzed through patient-specific computational fluid dynamics.
Article Title: Hemodynamic characterization of spontaneous isolated superior mesenteric artery dissection revealed by patient-specific computational fluid dynamics
Article References:
Wei, R., Chen, Z. Hemodynamic characterization of spontaneous isolated superior mesenteric artery dissection revealed by patient-specific computational fluid dynamics.
BioMed Eng OnLine 24, 101 (2025). https://doi.org/10.1186/s12938-025-01434-0
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12938-025-01434-0
Tags: blood flow patterns in arterieshemodynamic landscape analysishigh-resolution CT angiography applicationsinnovative treatment strategies for SISMADoscillatory shear index in dissectionpatient-specific computational fluid dynamicspersonalized diagnostics in vascular diseasesspontaneous isolated superior mesenteric artery dissectionsuperior mesenteric artery dissectionthree-dimensional CFD simulationsvascular emergencies researchwall shear stress measurements